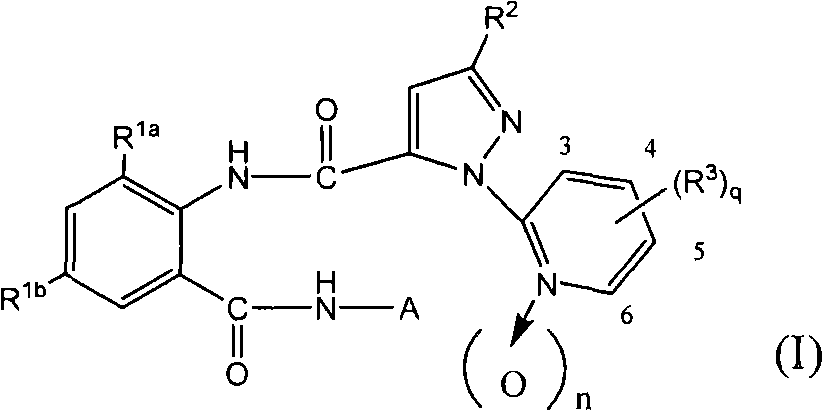
Pesticidal compositions
A pesticide composition and the technology of the composition, which are applied in the directions of biocides, animal repellents, arthropodicides, etc., can solve the problem of unsatisfactory pest effects, impossible to obtain satisfactory pesticidal effects, and low residual activity. effect, etc., to achieve the effect of stable insecticidal effect and high insecticidal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0505] Now, the present invention is described with reference to Examples, but it should be understood that the present invention is not limited thereto.
[0506] First, typical examples and production examples of the compound of formula (I) are described.
preparation Embodiment 1
[0508] N-[2-bromo-4-chloro-6-[[α-methyl-(cyclopropylmethyl)amino]carbonyl]-phenyl]-3-bromo-1-(3-chloro-2-pyridine base)-1H-pyrazole-5-carboxamide (compound No. 1) preparation
[0509]To a mixed liquid containing 0.6 g of α-methyl-cyclopropylmethylamine hydrochloride and 40 ml of tetrahydrofuran, 1 g of triethylamine was slowly added dropwise under ice-cooling, followed by stirring at room temperature for 1 hour. Then slowly add 0.85g of 2-[3-bromo-1-(3-chloro-2-pyridyl)-1H-pyrazol-5-yl]-6-chloro-8-bromo-4H-3 , a mixture of 1-benzoxazin-4-one and 10ml tetrahydrofuran. After the dropwise addition was terminated, the mixed solution was reacted under reflux for 4 hours. After the reaction was completed, the solvent was distilled off under reduced pressure, and ethyl acetate and water were added to the residue for extraction. The organic layer was washed with water and a saturated aqueous sodium chloride solution, and dried by adding anhydrous magnesium sulfate. The solvent was...
preparation Embodiment 2
[0511] N-[2-bromo-4-chloro-6-[[(cyclopropylmethyl)amino]carbonyl]-phenyl]-3-bromo-1-(3-chloro-2-pyridyl)-lH- Preparation of pyrazole-5-carboxamide (compound number 2)
[0512] The desired product having a melting point of 196-199° C. was obtained in the same manner as in Preparation Example 1, except that cyclopropylmethylamine hydrochloride was used instead of α-methylcyclopropylmethylamine hydrochloride.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com