Means and methods of enhancing delivery to biological systems
A guanidine group and carboxyl group technology, which can be applied in the directions of urinary system diseases, endocrine system diseases, nano-drugs, etc., can solve the problems of failure to provide, achieve economical production, and avoid allergic or immunogenic effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
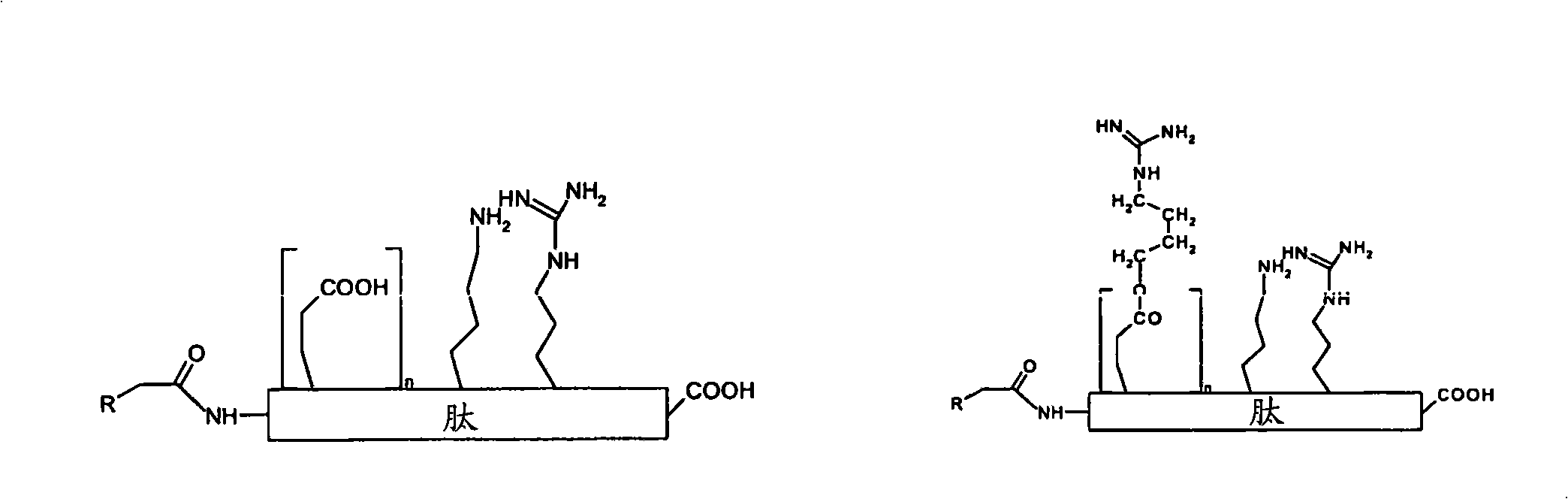
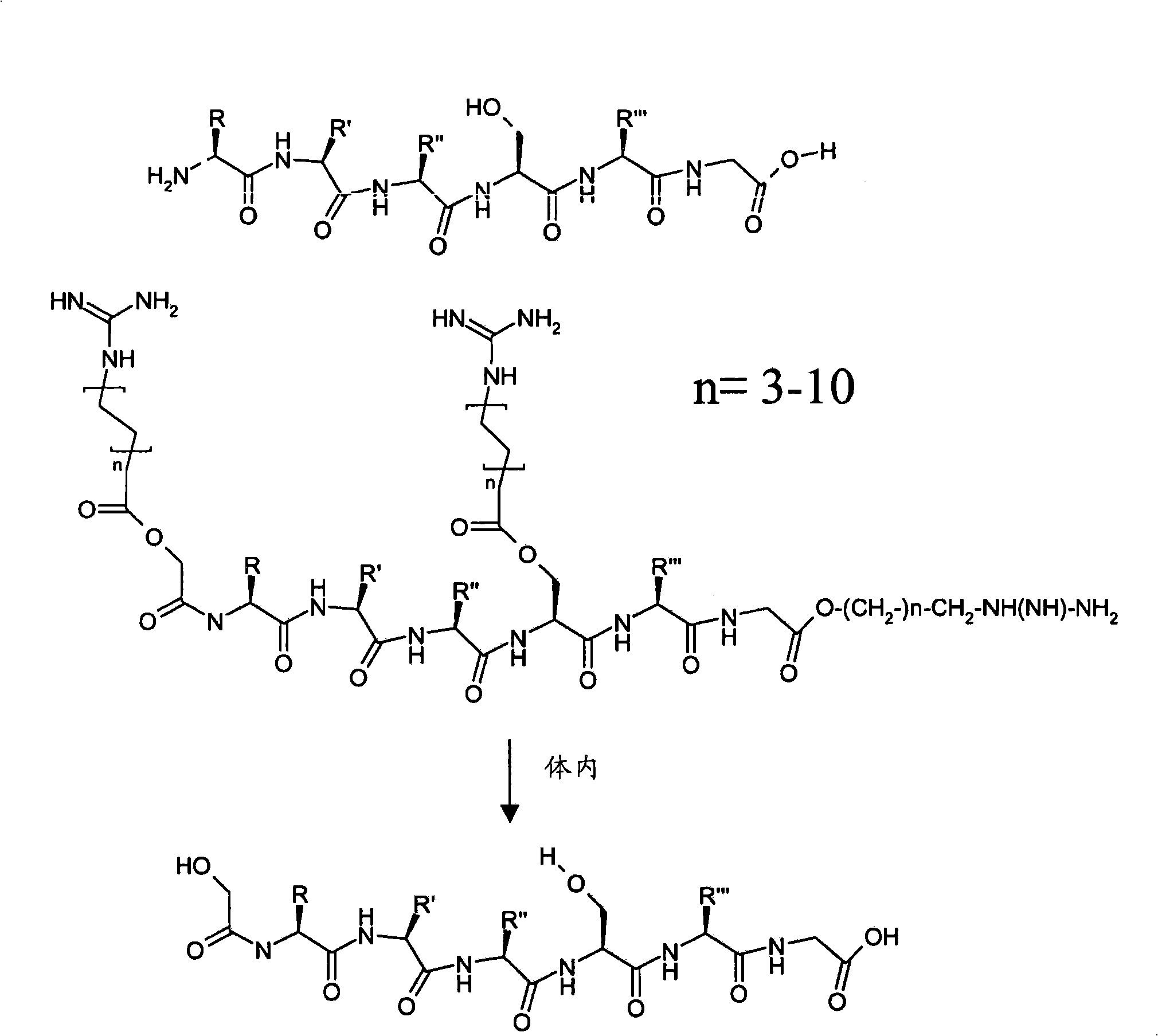
[0168] In the absence of gene fusion (ie, cell penetrating) peptides, SCF (stem Delivery of 20-mer peptide inhibitors of cytokines)
[0169] Target peptide (required for ER export of SCF):
[0170] N-E-E-D-N-E-I-S-M-L-Q-E-K-E-R-E-F-Q-E-V-cooH
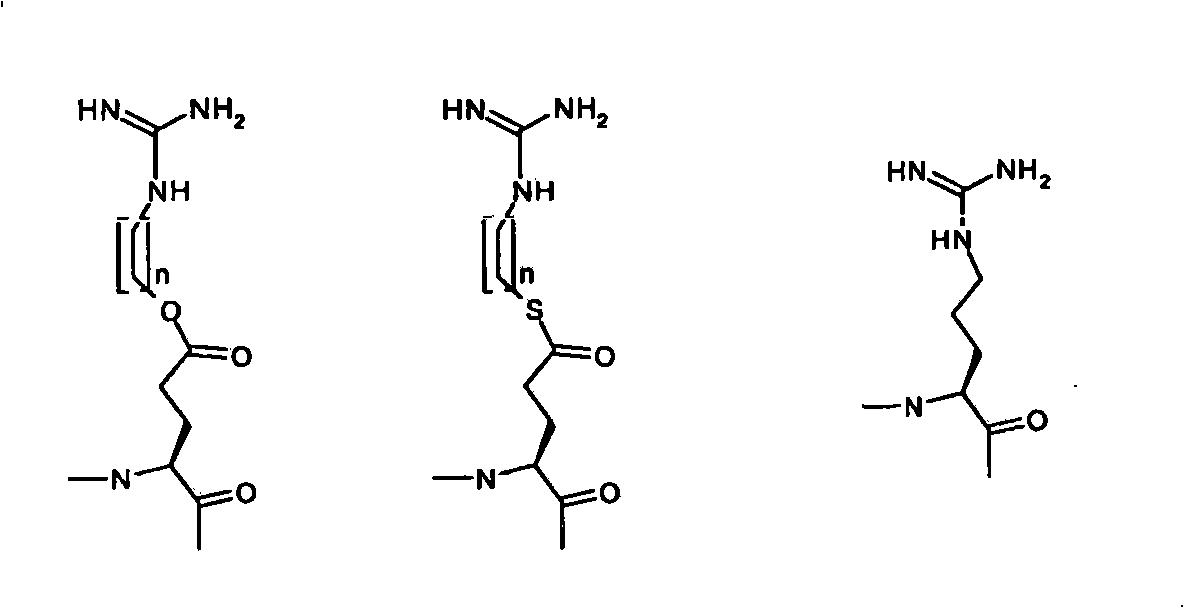
[0171] the corresponding arginine-ester-peptide, which is modified according to the invention by esterification of the carboxyl group of glutamic acid with guanidinol;
[0172]
[0173] Gnd = guanidino
[0174] The aspartic acid side chain is protected with a methyl ester: Boc-Asp(OMe)-OH.
[0175] I. Pseudo-arginine-derived SFC derivatives:
[0176] The guanidine group is chemically introduced into the side chain of glutamic acid via an ester bond, separated from the carboxylic acid by a 4-carbon alkyl chain (butyl). The guanidino protecting group is bis-Z(N,N'dibenzyloxycarbonyl).
[0177] Strategy: Boc / Fmoc strategy.
[0178] Precursor used: Fmoc-Glu(ObutylguanidinobisZ(bisZ))-OH.
[0179] Resin: Boc-Val-Pam resin RAPP ...
Embodiment 2
[0191] Synthesis of 1-(2-(benzyloxy)ethyl)-N,N'-di-Boc-guanidine
[0192]49.9 mg (1 mmol, 1 eq.) of NaH were suspended in 10 mL of dry THF under nitrogen. A solution of 298.3 mg (1 mmol, 1 eq.) of 1-(6-hydroxyethyl)-N,N'-di-Boc-guanidine in 10 mL of dry THF was added dropwise over 10 minutes. The solution was stirred at room temperature for 1 hour and 0.12 mL (1 mmol, 1 eq.) of benzyl bromide was added dropwise. The reaction was stirred at room temperature for 2 hours and 30 minutes and checked by TLC. 44.9 mg (1 mmol, 1 eq.) of NaH were added. After 5 hours 30 minutes (total reaction time), TLC indicated little progress and the reaction was allowed to stir overnight. Some water was added and the organic solvent was removed under vacuum. The aqueous phase was extracted three times with ether. Organic phase in anhydrous Na 2 SO 4 Drying and evaporation gave 430.0 mg of a colorless oil. The oil was subjected to flash chromatography on silica (eluent AcOEt:pentane 1:9...
Embodiment 3
[0194] 2-Guanidinoethylbenzoic acid trifluoromethanesulfonate Synthesis of (2-guanidinoethyl benzoate triflate salt)
[0195] 4mL (52mmol, 65eq.) of TFA was added to 315.5mg (0.8mmol, 1eq.) of 1-(2-(benzyloxy)ethyl)-N,N'-di-Boc-guanidine and at room temperature Stir for 3 hours. The solution was evaporated to obtain 336.9 mg (1.1 mmol) of 2-guanidinoethylbenzoic acid triflate as a pale orange oil (yield = 110%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com