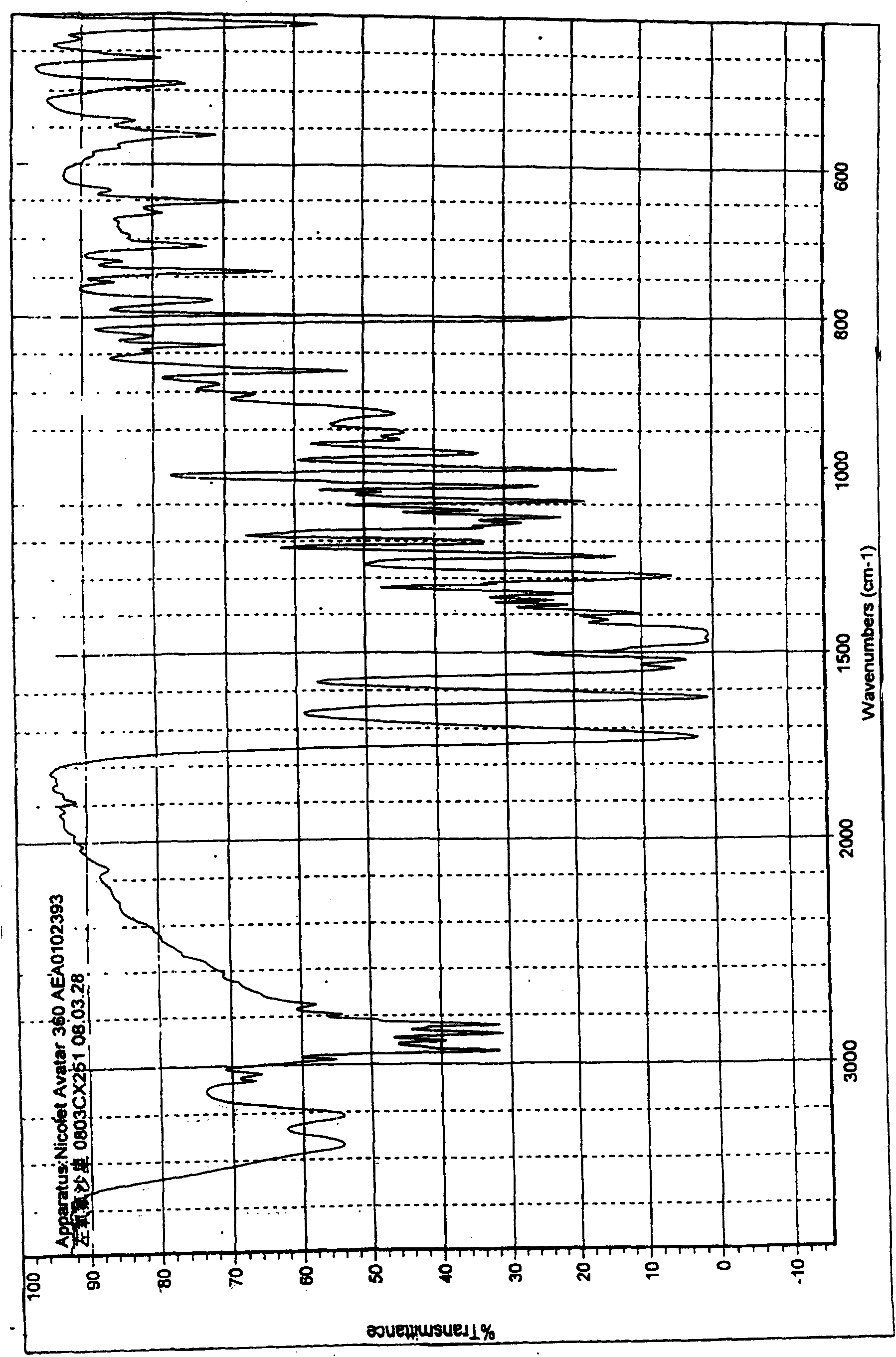
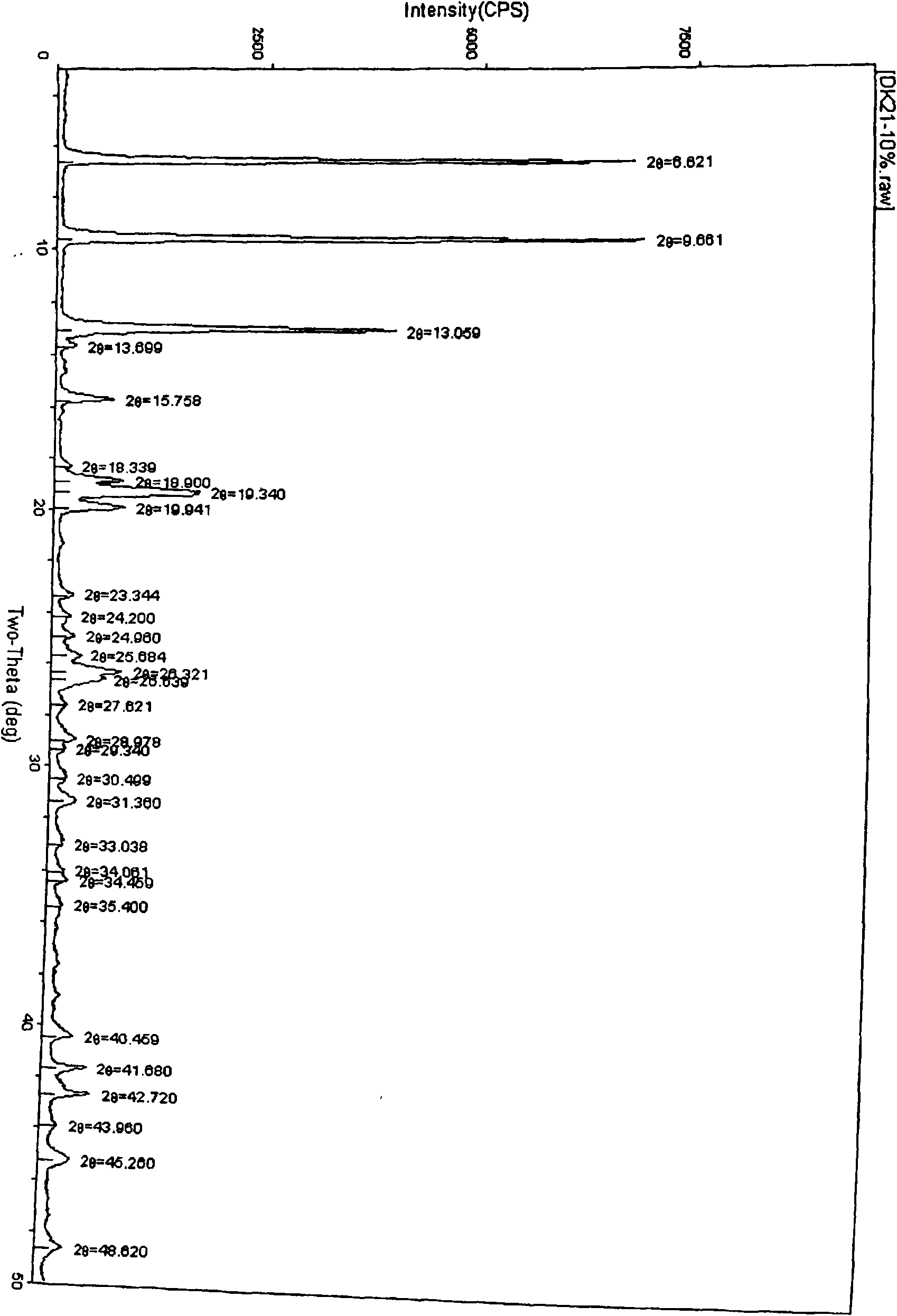
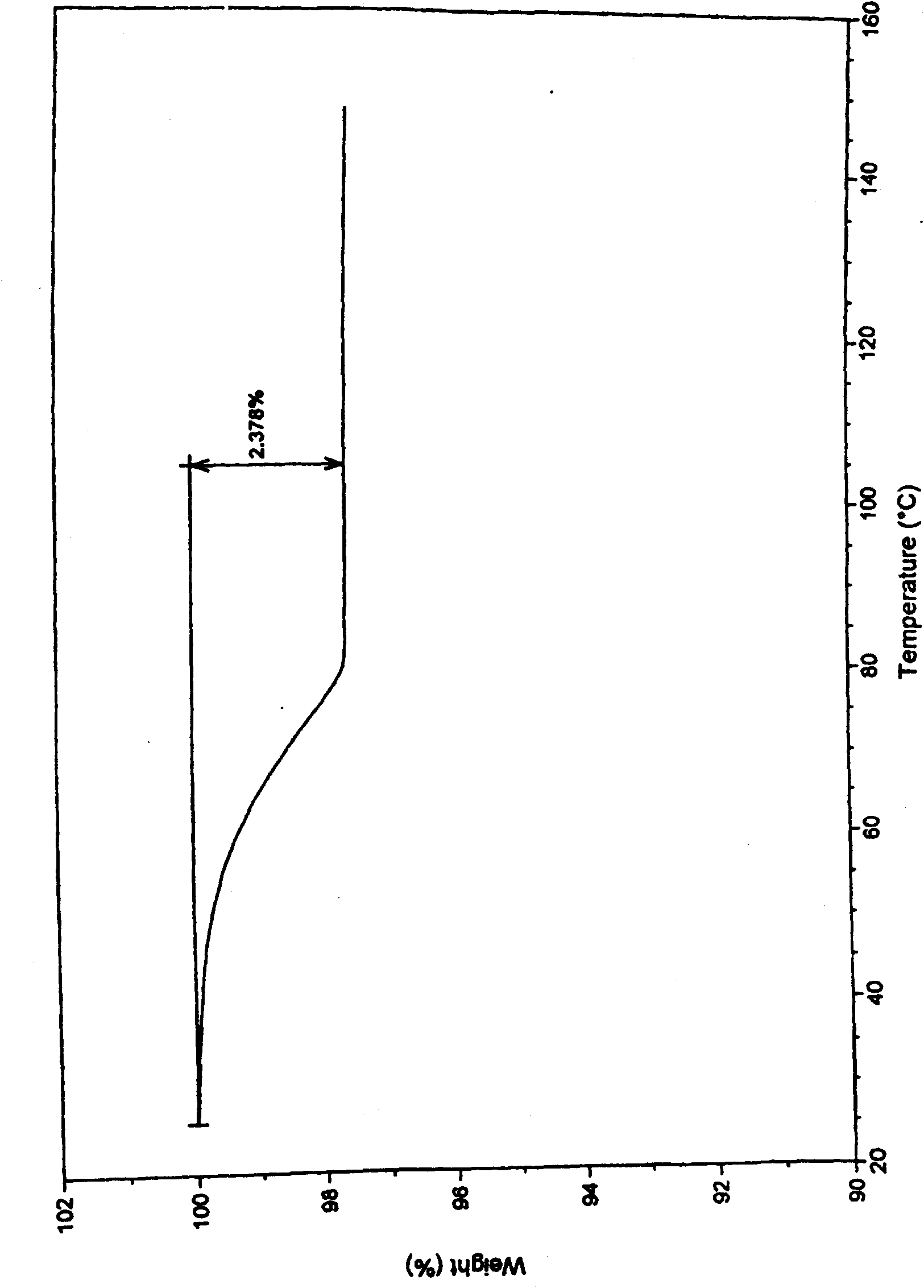
Method for preparing high-purity pseudo-polymorphic lavo-ofloxacin hemihydrate
A technology of levofloxacin and hydrate, which is applied in the field of preparation of high-purity polymorphic levofloxacin hemihydrate, can solve the problems of unfavorable quality and yield of finished levofloxacin hemihydrate, difficult microbial degradation, complicated operation procedures, etc. The effect of recovery, good solubility and simple operation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1 ethanol: water (85: 15, w / w)
[0027] Put 20g of levofloxacin crude product into a reaction bottle equipped with a reflux device, then add 170g of ethanol and 30g of water, add 2g of activated carbon after heating and dissolving, reflux for decolorization for 45 minutes, suction filter while it is hot, and use 15g of alcohol with a moisture content of 15%. (w / w) Rinse the carbon cake, combine the filtrates, cool down and crystallize under stirring conditions, vacuum filter, rinse the product with 15g of refrigerated alcohol (w / w) with a moisture content of 15%, and vacuum dry at 60-65°C for 8 hours , 17.3 g (86.5%) of pseudopolymorph levofloxacin hemihydrate was obtained.
Embodiment 2
[0028] Embodiment 2 methanol: water (90: 10, w / w)
[0029] Put 20g of levofloxacin crude product into a reaction flask equipped with a reflux device, then add 180g of ethanol and 20g of water, add 2g of activated carbon after heating and dissolving, reflux for decolorization for 45 minutes, suction filter while it is hot, and use 15g of methanol with a moisture content of 10%. (w / w) Rinse the carbon cake, combine the filtrates, cool down and crystallize under stirring conditions, vacuum filter, rinse the product with 15g of frozen methanol (w / w) with a moisture content of 10%, and vacuum dry at 60-65°C for 8 hours , 16.9 g (84.5%) of pseudopolymorphic levofloxacin hemihydrate was obtained.
Embodiment 3
[0030] Embodiment 3 Ethanol: tetrahydrofuran: water (70: 15: 15, w / w / w)
[0031] Put 20g of levofloxacin crude product into a reaction bottle equipped with a reflux device, then add 140g of ethanol, 30g of tetrahydrofuran and 30g of water, heat and dissolve, then add 2g of activated carbon, reflux for decolorization for 45 minutes, suction filter while it is hot, and use 15g of the same proportioning Rinse the charcoal cake with a mixed solvent, combine the filtrates, cool down and crystallize under stirring conditions, vacuum filter, rinse the product with 15 g of the frozen mixed solvent, and vacuum dry for 10 hours at 55-60 ° C to obtain 17.0 g (85.0%) of pseudopolymorphs type levofloxacin hemihydrate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com