Robust sustained release formulations
A sustained-release preparation and sustained-release technology, applied in the field of sustained-release pharmaceutical preparations, can solve the problems of low oral bioavailability and short duration of action of immediate-release oxymorphone
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
other Embodiment approach
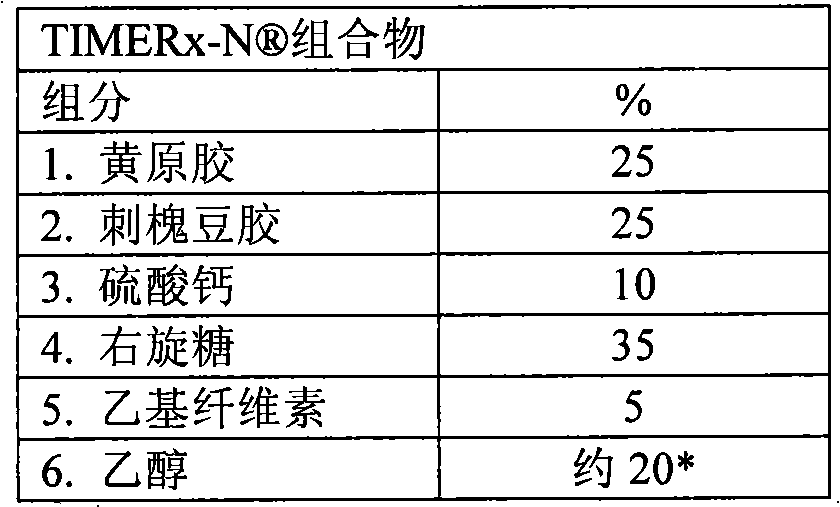
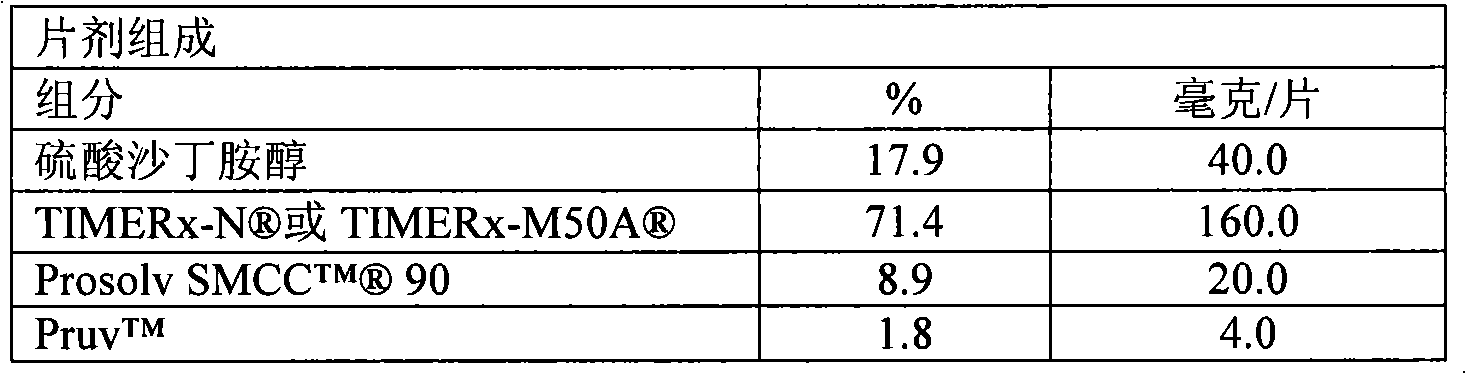
[0177] Other embodiments provide a drug comprising about 1-200 mg of oxymorphone hydrochloride, or about 5-80 mg of oxymorphone hydrochloride; and about 80-200 mg of an extended release delivery system, or about 120-200 mg of an extended release delivery system, or about 160 A robust sustained release solid dosage form formulation of a milligram sustained release delivery system; wherein the sustained release delivery system comprises about 8.3-41.7% locust bean gum, or about 25% locust bean gum; at least about 30% of the xanthan gum particles are capable of About 25-41.7% xanthan gum that passes through a #270 mesh sieve, or about 25% xanthan gum that at least about 30% of the particles are able to pass through a #270 mesh sieve; about 20-55% dextrose, or about 35% dextrose sugar; about 5-20% calcium sulfate dihydrate, or about 10% calcium sulfate dihydrate; and about 2-10% ethylcellulose, or about 5% ethylcellulose.
[0178] Some embodiments provide a drug comprising about 1...
other Embodiment approach
[0179] Other embodiments provide a drug comprising about 1-200 mg oxymorphone hydrochloride, or about 5-80 mg oxymorphone hydrochloride; and about 200-420 mg sustained release delivery system, or about 300-420 mg sustained release delivery system, or about 360 mg A robust sustained release solid dosage form formulation of a milligram sustained release delivery system; wherein the sustained release delivery system comprises about 8.3-41.7% locust bean gum, or about 25% locust bean gum; at least about 30% of the xanthan gum particles are capable of About 8.3-41.7% xanthan gum that passes through #270 mesh, or about 25% xanthan gum that at least about 30% of the particles are able to pass through #270 mesh; about 20-55% dextrose, or about 35% dextrose sugar; about 5-20% calcium sulfate dihydrate, or about 10% calcium sulfate dihydrate; and about 2-10% ethylcellulose, or about 5% ethylcellulose.
[0180] When administered orally, the patient's robust sustained-release formulations...
Embodiment 1
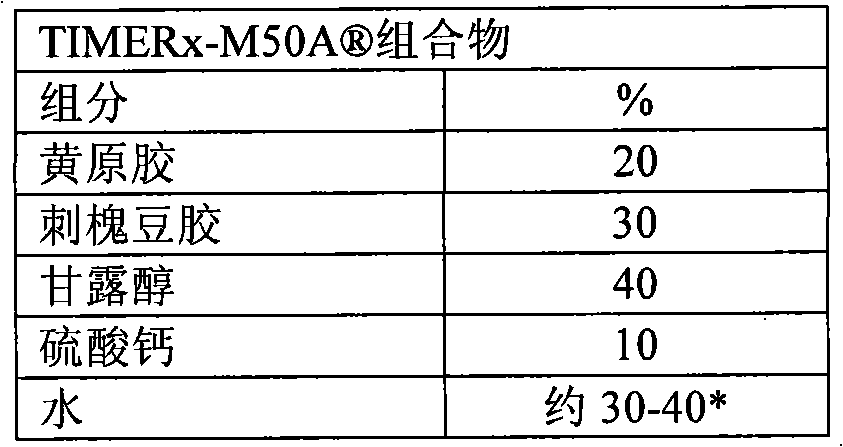
[0211] Prepare TIMERx- Sustained Release Delivery System
[0212] Batches of TIMERx- Sustained release delivery system.
[0213]Batches of xanthan gum (Jungbunzlauer, Perhoven, Austria or CP Kelco, Chicago, IL) were tested for particle size using a series of mesh screens. These mesh screens include #270 mesh screens which allow particles less than 53 microns in diameter to pass through (fines). The weight fraction of xanthan gum particles passing through the mesh sieve (ie fraction of xanthan gum fines) was determined. Batches with known xanthan fines weight fractions were then prepared. Dry blend the necessary amounts of xanthan gum, locust bean gum, calcium sulfate and dextrose in a high speed mixer / granulator for 3 minutes to prepare TIMERx- A hydrophobic polymer (ethylcellulose) slurry was prepared by dissolving ethylcellulose in ethanol. The slurry was added to the above dry blend mixture and the material was pelletized for 4 minutes with the chopper / impeller run...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com