Tablet capsule containing clopidogrel hydrogen sulfate salt tablets and acetylsalicylic acid tablets
A technology of acetylsalicylic acid tablets and bisulfate tablets, which is applied in the direction of medical preparations containing active ingredients, capsule delivery, organic active ingredients, etc., to achieve the effect of improving stability and enhancing compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
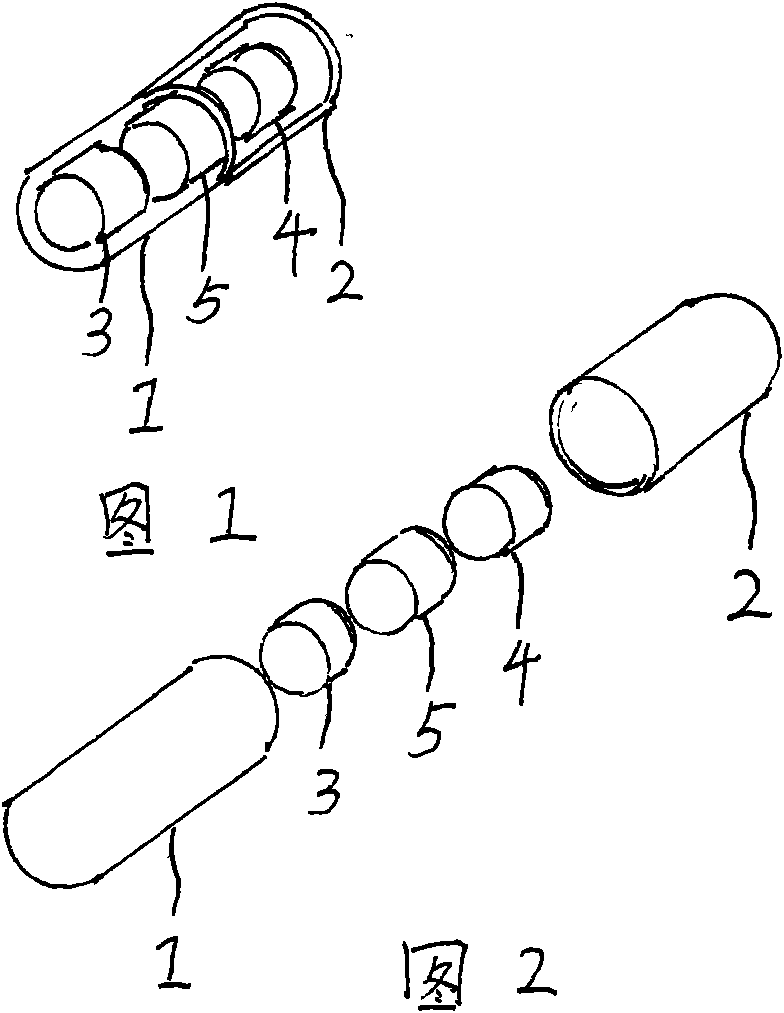
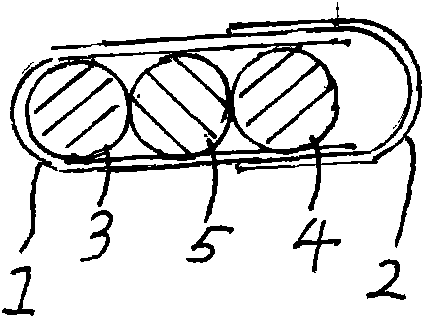
[0037] Embodiment 1: see attached figure 1 As shown, a capsule filled with clopidogrel bisulfate tablets, acetylsalicylic acid tablets and starch tablets. The capsule specification is size 0. The capsule is composed of a capsule shell and a filling. The capsule shell is composed of a lower capsule body 1 and an upper capsule body 2. The filling is three cylindrical tablets of different colors. Gray bisulfate tablet 3 (containing clopidogrel 75 mg), acetylsalicylic acid tablet 4 (containing acetylsalicylic acid 75 mg) and the third tablet (starch tablet) 5.
Embodiment 2
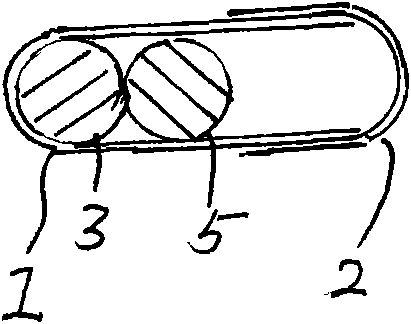
[0038] Embodiment 2: see attached Figure 4 As shown, a capsule filled with clopidogrel bisulfate tablets and acetylsalicylic acid tablets. The capsule specification is size 0. The capsule is composed of a capsule shell and a filling. The capsule shell is composed of a lower capsule body 1 and an upper capsule body 2. The filling is two cylindrical tablets of different colors. The tablets are film-coated tablets of different colors, respectively. Clopidogrel bisulfate tablet 3 (containing clopidogrel 75mg) and acetylsalicylic acid tablet 4 (containing acetylsalicylic acid 75mg).
Embodiment 3
[0039] Example 3: The control drug capsule was prepared by the simple mixing method in Example 6 of Chinese Patent CN1359294A. It contains 75mg of clopidogrel and 75mg of acetylsalicylic acid.
[0040] Stability comparison: The samples obtained in Examples 1-3 were respectively sampled and measured at 0, 5, and 10 days under high temperature (40°C) and high humidity (RH75%) conditions, and the influencing factors were investigated. The results are shown in Table 1 and table 2:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com