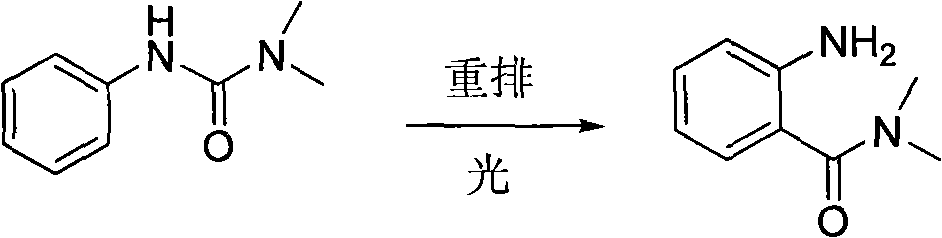
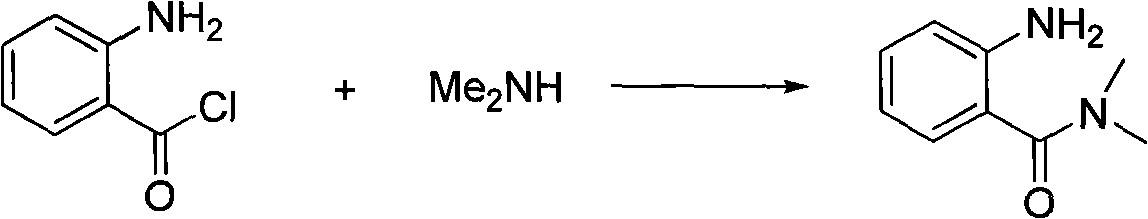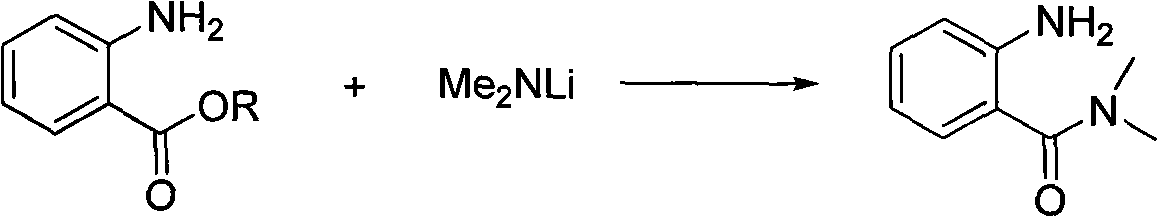Preparing method of N, N-dimethylbenzamide
A technology of dimethylbenzamide and dimethylamine, which is applied to the preparation of carboxylic acid amides, the preparation of organic compounds, chemical instruments and methods, etc., and can solve the problem of harsh preparation and storage conditions and hydrogenation process of dimethylamine lithium salt Problems such as poor operation safety and difficult preparation of raw materials have achieved the effect of cheap and easy-to-obtain raw materials, high safety and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1 o-amino-N, the preparation of N-dimethylbenzamide
[0021] Take 82.3g (0.5mol, 99.0%) of isatoic anhydride in a reaction flask, add 400mL of methanol, start stirring, cool down to below -10°C with frozen brine, and then introduce 34g (0.75mol, 99.9%) of dimethylamine gas . Subsequently, it was returned to room temperature, and the reaction was stirred for 4 hours. Negative pressure precipitation yielded 80.5 g of the target compound o-amino-N,N-dimethylbenzamide with a purity of 98.8% and a yield of 96.8%.
Embodiment 2
[0022] Embodiment 2 o-amino-N, the preparation of N-dimethylbenzamide
[0023] Take 82.3g (0.5mol, 99.0%) of isatoic anhydride in a reaction flask, add 400mL of dichloroethane, start stirring, cool down to below 10°C with frozen brine, then add 68g (0.6mol, 40%) of dimethyl Amine solution. Subsequently, it was returned to room temperature, and the reaction was stirred for 5 hours. Separate the layers, extract the aqueous phase once with dichloroethane, combine the organic phases, and precipitate under negative pressure to obtain 74.2 g of the target compound o-amino-N, N-dimethylbenzamide, with a purity of 94.0% and a yield of 85.0 %.
Embodiment 3
[0024] Embodiment 3 o-amino-N, the preparation of N-dimethylbenzamide
[0025] Take 82.3g (0.5mol, 99.0%) of isatoic anhydride in a reaction flask, add 400mL of acetonitrile, start stirring, cool down to below 10°C with frozen brine, then add 62g (0.55mol, 40%) of dimethylamine aqueous solution. Subsequently, it was returned to room temperature, and the reaction was stirred for 4 hours. Negative pressure precipitation yielded 77.8 g of the target compound o-amino-N,N-dimethylbenzamide with a purity of 96.5% and a yield of 91.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com