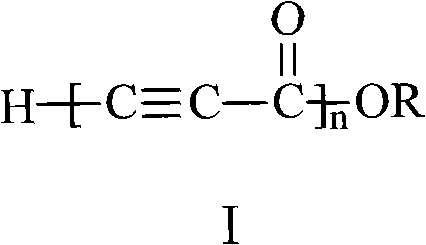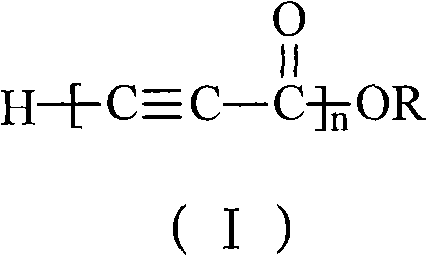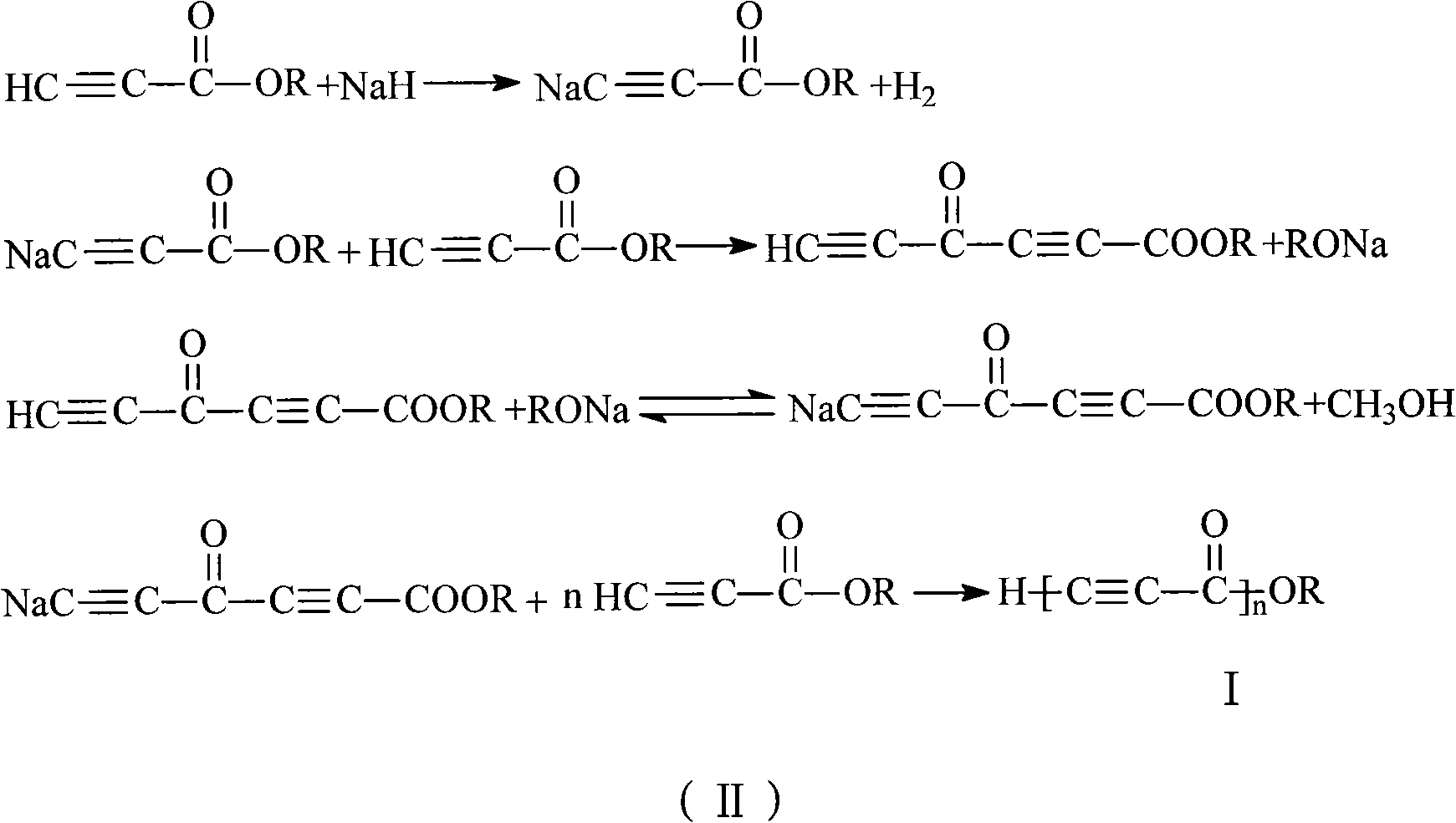Alkynyl-containing conjugated polymers and synthetic method thereof
A technology of conjugated polymers and synthetic methods, applied in the field of alkyne-containing conjugated polymers and their synthesis, can solve the problems of low reaction yield, low monomer conversion rate, wide product molecular weight distribution, etc., and achieve solubility Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Add 1ml of monomeric methyl propiolate into the two horn tubes with a 1ml syringe, then put the two horn tubes into the atmosphere at -20°C, and add 139.5 mg of initiator sodium hydride (the ratio of the amount of the monomer to the initiator substance 2:1), reacted for 24 hours to obtain a black solid; washed the black solid with dilute hydrochloric acid with a mass concentration of 25% for 0.5 hours, separated to obtain a solid product, and dried it in a vacuum oven at 50°C to obtain a conjugated compound containing an alkyne group polymer. The conversion rate of monomer was 44.3%.
Embodiment 2
[0025] Add 1ml of monomeric methyl propiolate into the two horn tubes with a 1ml syringe, then put the two horn tubes into the atmosphere at -20°C, and add 2.8 mg of initiator sodium hydride (the ratio of the amount of the monomer to the initiator substance 100:1), reacted for 24 hours to obtain a black solid; the black solid was washed with dilute hydrochloric acid with a mass concentration of 25% for 0.5 hours, and the solid product was isolated, and dried in a vacuum oven at 50°C to obtain a conjugated alkyne group polymer. The conversion rate of monomer was 39.2%.
Embodiment 3
[0027] Add 1ml of monomeric methyl propiolate into two mouths of horn tubes with a 1ml syringe, then put the two mouths of horn tubes into an atmosphere of 30°C, and add 9.3 mg of initiator sodium hydride (the molar ratio of the monomer and the initiator substance is 30:1), reacted for 6 hours to obtain a black solid; washed the black solid with dilute hydrochloric acid with a mass concentration of 25% for 0.5 hours, separated to obtain a solid product, and dried it in a vacuum oven at 50°C to obtain things. The conversion rate of monomer was 78.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com