Application of zidovudine lipid derivative in medicaments for treating virus associated diseases
A zidovudine and virus technology, which is applied in the application field of zidovudine lipid derivatives in the treatment of virus-related diseases, and can solve problems such as drug resistance or drug resistance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
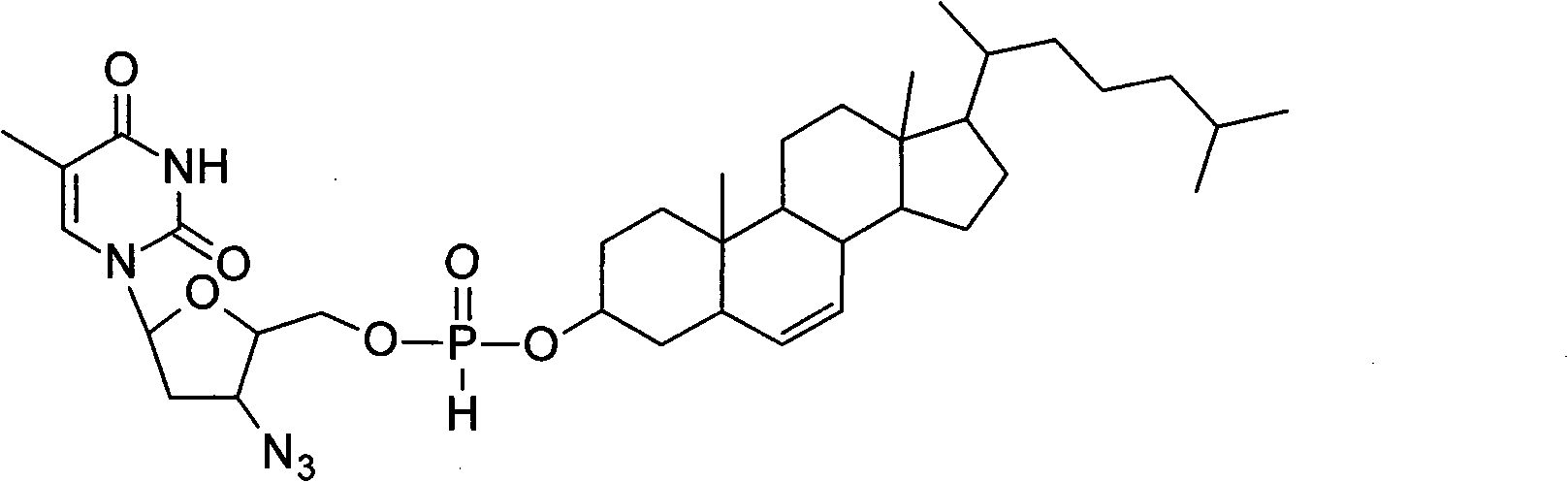
[0033] Experimental example 1: Anti-HIV activity of cholesteryl phosphonozidovudine in vitro
[0034] Cholesterylphosphonozidovudine has strong inhibitory activity against HIV-1 IIIB virus in vitro, and has low cytotoxicity.
[0035] Materials and Methods
[0036] Routine instruments, equipment and reagents for cell and HIV virus experiments are used in the P3 laboratory. Zidovudine raw materials are purchased from pharmaceutical factories. The preparation of the cholesterol-based phosphonozidovudine self-assembly delivery system refers to the aforementioned patent (CN101244036), with an average particle size of 107 nanometers and stability. The HIV-1 IIIB virus was imported from the United States; the MT4 cell line was imported from Japan.
[0037] The concentration of zidovudine aqueous solution and self-made cholesterylphosphonozidovudine self-assembled delivery system measured by high performance liquid chromatography were 3.74mol L -1 , 3.03mol·L -1 , both were dilut...
experiment example 2
[0050] Experimental Example 2: In Vitro Anti-HIV Activity of Cholesteryl Phosphoryl Zidovudine
[0051] Cholesteryl phosphoryl zidovudine has strong inhibitory activity against HIV-1 IIIB virus in vitro, and has low cytotoxicity.
[0052] Materials and Methods
[0053] Similar to Experimental Example 1, the preparation of the cholesterol-based phosphoryl zidovudine self-assembly delivery system refers to the aforementioned patent (CN100462103), with an average particle size of 123 nanometers and stability.
[0054] The concentrations of zidovudine aqueous solution and cholesteryl phosphoryl zidovudine self-assembled delivery system measured by high performance liquid chromatography were 3.74mol L -1 , 2.22mol·L -1 , both were diluted with ultrapure water to about 50 μmol L -1 , in a 96-well cell culture plate, the three drugs were serially diluted 10 times to obtain a concentration of 50×10 3 , 5×10 3 , 500, 50, 5, 0.5nmol·L -1 Zidovudine aqueous solution, cholesterol-ba...
experiment example 3
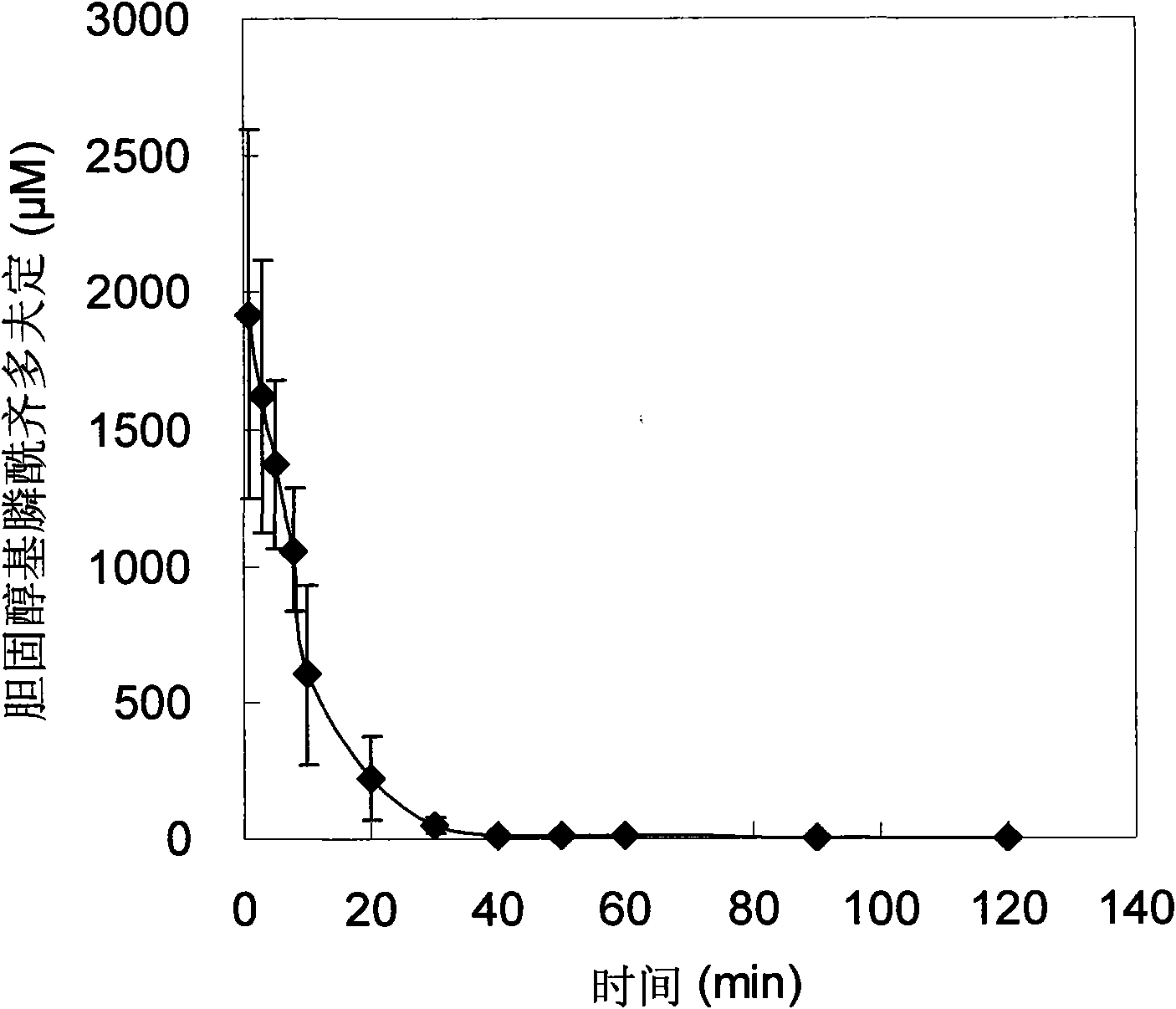
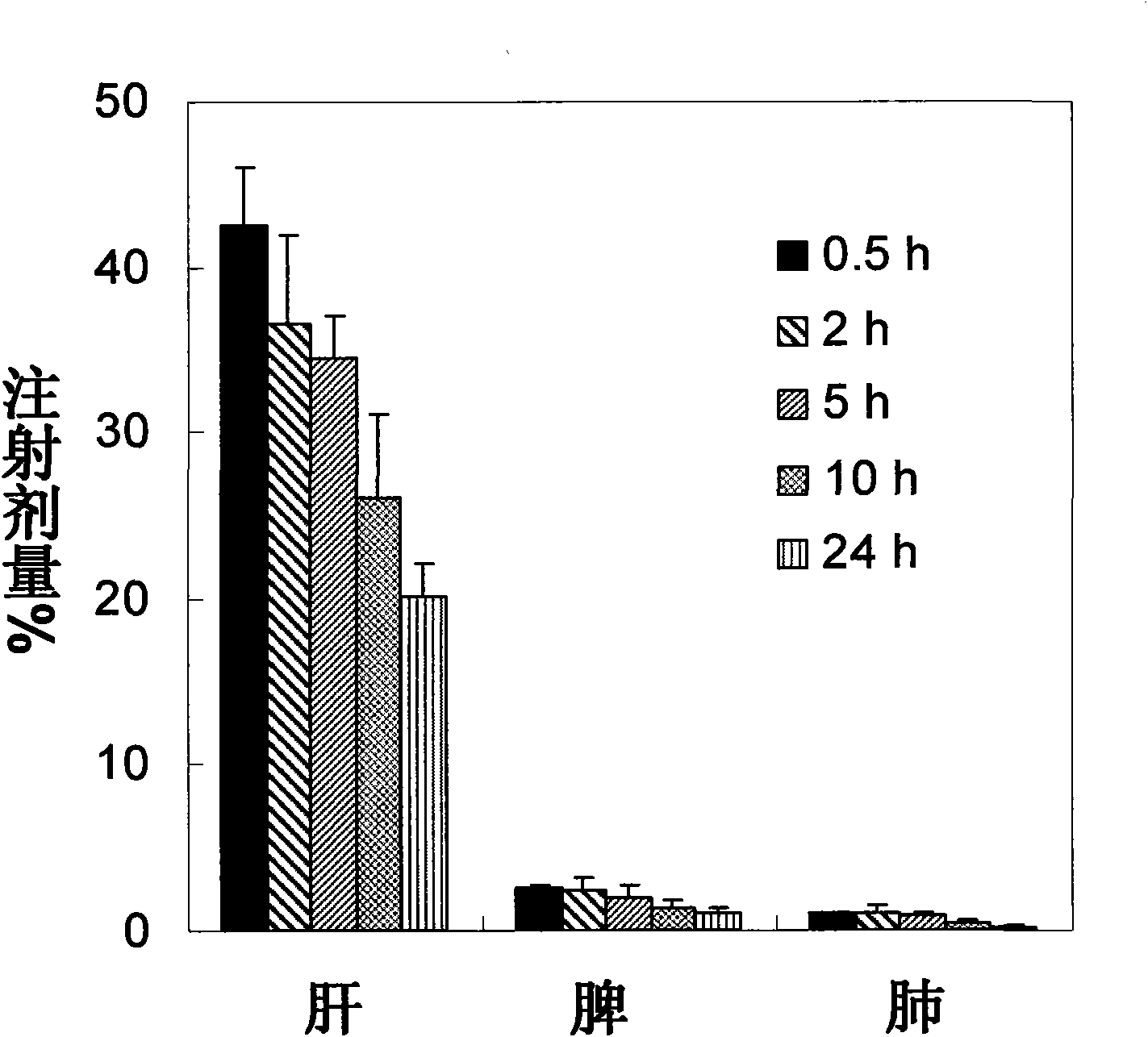
[0063] Experimental Example 3: Pharmacokinetics and tissue distribution of cholesteryl phosphonozidovudine self-assembled delivery system after intravenous injection
[0064] Cholesterol-based phosphonozidovudine self-assembled delivery system can be quickly cleared from the blood circulation after intravenous injection to rats, targeted distribution to the mononuclear macrophage system such as liver, lung and spleen, and has a strong macrophage Cell targeting provides the basis for efficient clearance of viruses in monocytes and macrophages.
[0065] Materials and Methods
[0066] Cholesterol-based phosphonozidovudine self-assembled delivery system, concentrated to about 20 mg / ml, filtered and sterilized through a 0.22 μm sterile filter membrane before administration. Samples before administration were all accurately determined drug content by HPLC method. The rest are reagents and equipment for routine animal experiments. Healthy male Sprague-Dawley adult rats.
[0067] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com