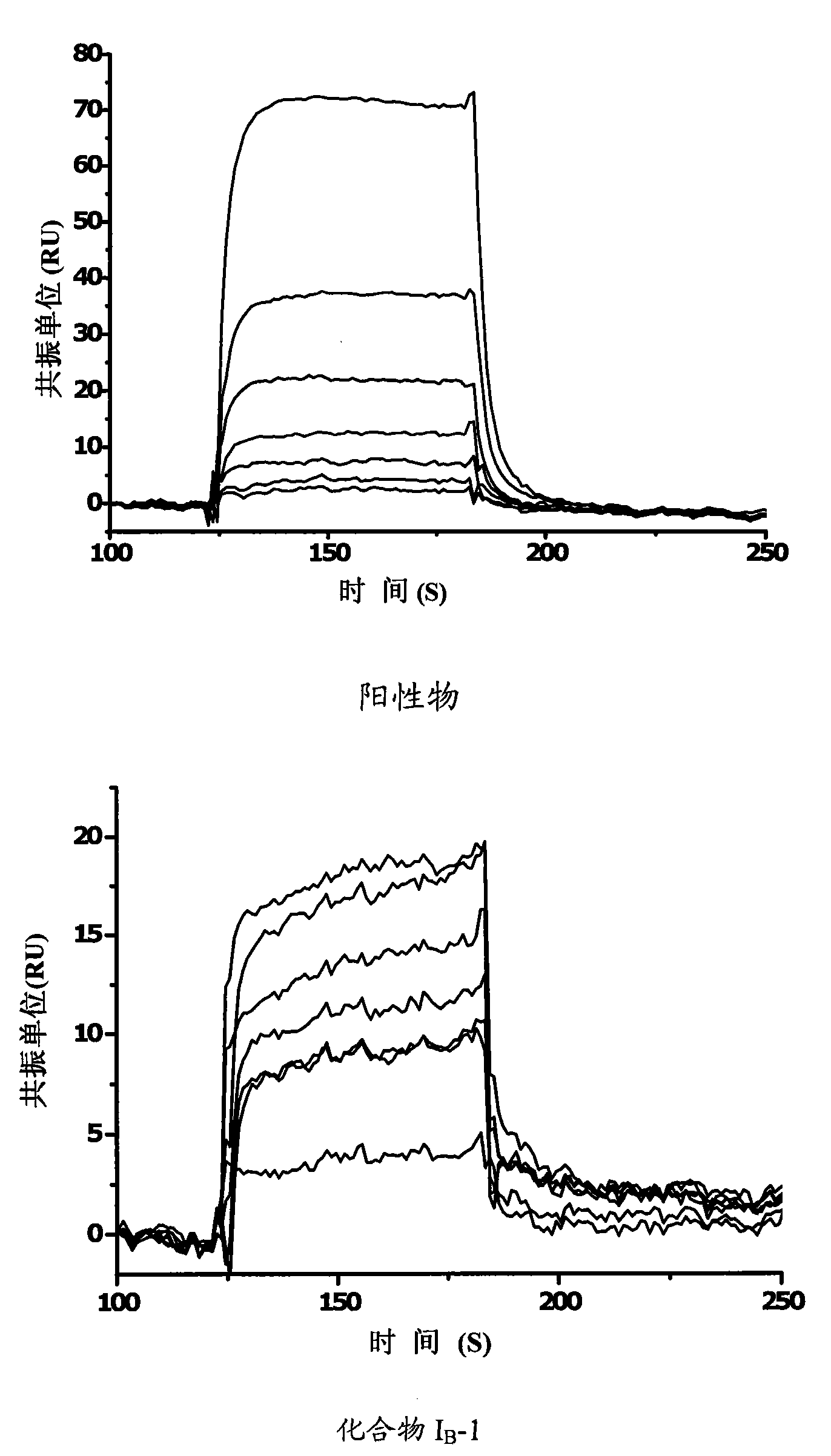
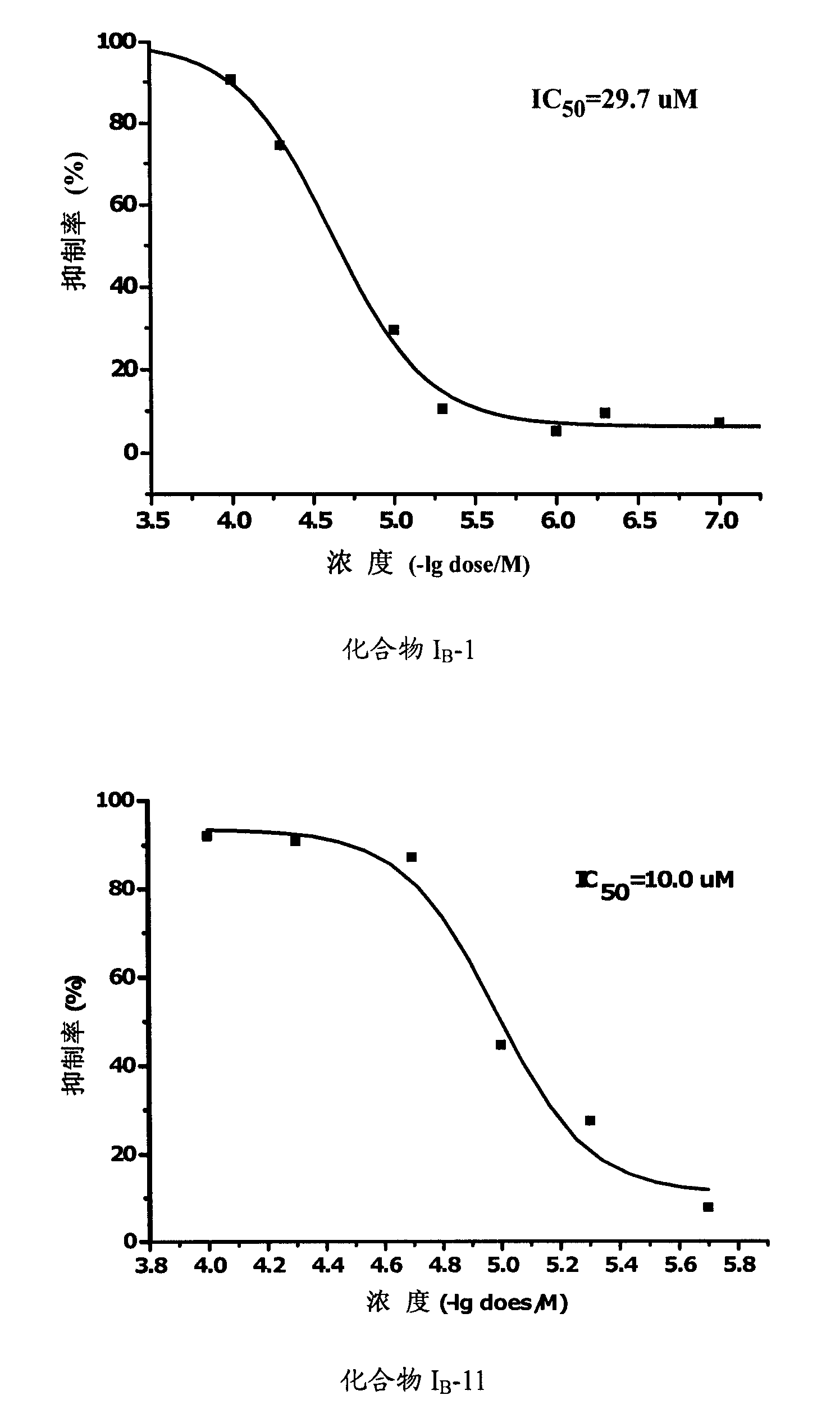
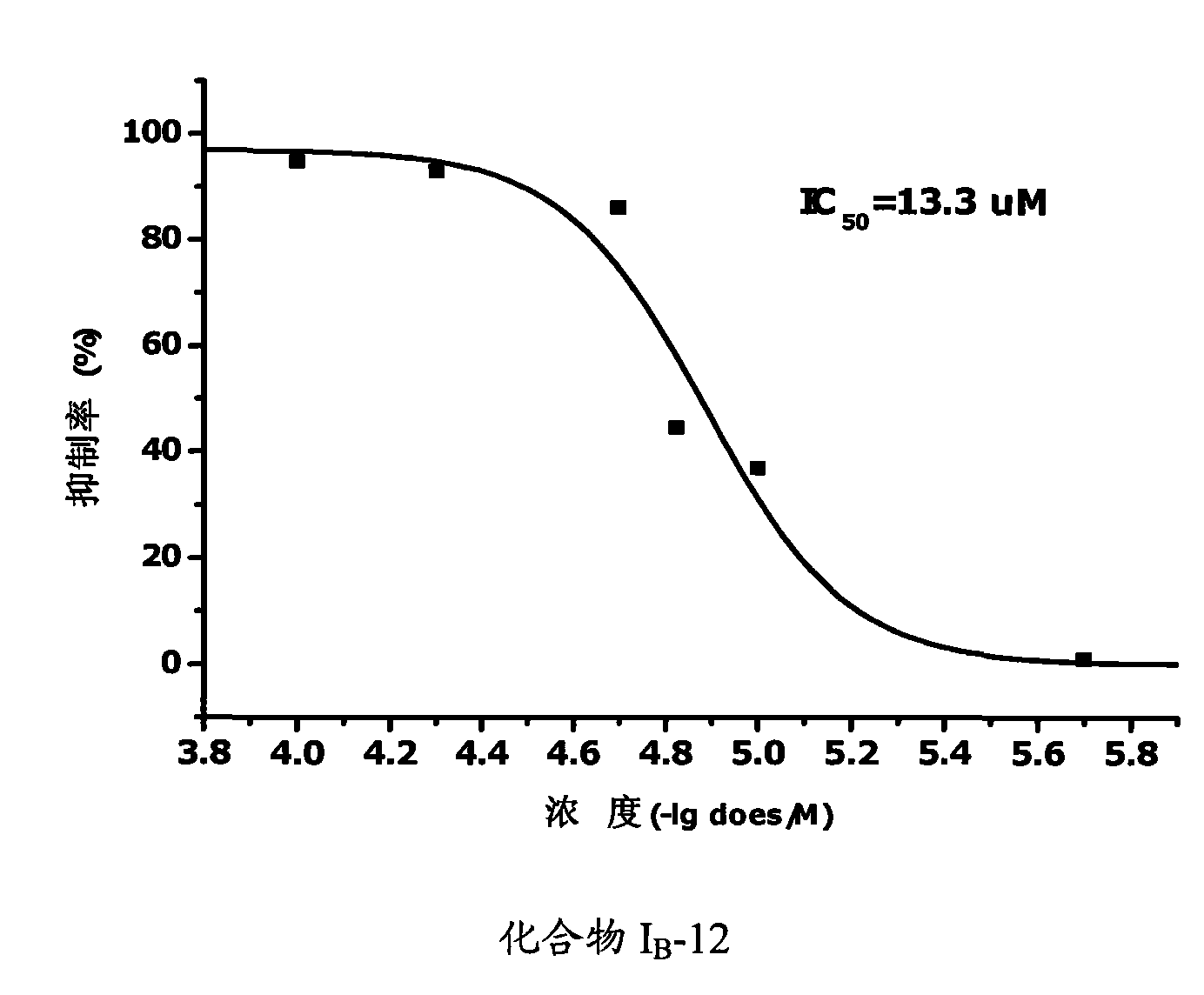
Propionamides compound and usage thereof
A propionamide and compound technology, applied in the field of propionamide compounds, can solve problems affecting the function of oocysts, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Preparation of N-benzyl-2-(R)-Bocamino-3-(1H-indol-3-yl)propionamide (compound II-1)
[0042] Mix 0.62 g of N-Boc-D-tryptophan, 0.42 g of 1-hydroxybenzotriazole, 0.59 g of diisopropylethylamine, 1.27 ml of N, N-diisopropylethylamine with 10 ml of dichloro Mix and stir methane at 0°C for 1 hour, add 0.22 ml of benzylamine dropwise, raise to 20°C-30°C and stir for 48 hours; wash the organic layer with dilute acid, dilute alkali, and water respectively, dry, evaporate the solvent to dryness, and the residue column layer Analysis and separation gave 0.68 g of white solid with a yield of 84.4%. mp 142-143°C; 1 H NMR (CDCl 3 , 500MHz) δ1.39(s, 9H), 3.16(m, 1H), 3.33(m, 1H), 4.26(m, 2H), 4.41(m, 1H), 6.96(m, 3H), 7.13(t , 1H), 7.18-7.21 (m, 4H), 7.35 (d, 1H), 7.66 (d, 1H).
Embodiment 2
[0044] Preparation of N-benzyl-2-(R)-amino-3-(1H-indol-3-yl)propionamide hydrochloride (compound III-1)
[0045] Add 0.68 g of II-1 and 5 ml of saturated hydrochloric acid methanol into 10 ml of tetrahydrofuran, stir overnight at 20°C-30°C; evaporate the solvent, and wash the residue with a small amount of a mixture of chloroform and methanol (20 / 1, V / V) , 0.5 g of a yellow solid was obtained, with a yield of 87.8%. mp 214-218°C; 1 H NMR (CDCl 3 , 500MHz) δ3.16(m, 1H), 3.25(m, 1H), 4.02(s, 1H), 4.27(m, 2H), 7.00(t, 1H), 7.07-7.10(m, 3H), 7.19 -7.27 (m, 4H), 7.38 (d, 1H), 7.66 (d, 1H).
Embodiment 3
[0047] N-benzyl-2-(R)-benzylamino-3-(1H-indol-3-yl)propionamide (compound I A -1) Preparation
[0048] Dissolve 0.25 g of III-1 in ether, add 10% potassium carbonate solution, separate the ether layer, extract the water layer with ether, combine the ether layers, dry over anhydrous sodium sulfate, and concentrate. Add 0.2 g of anhydrous magnesium sulfate and 5 ml of dichloromethane to the concentrate, add 0.06 ml of benzaldehyde dropwise at 20°C-30°C, stir at 20°C-30°C for 6 hours; wash with saturated sodium carbonate, and dry the organic layer; evaporate the solvent to dryness , the residue was added with appropriate amount of methanol and sodium borohydride, and stirred at 0°C for 1 hour to obtain 80 mg of yellow solid with a yield of 34%. mp 38-40°C; 1 H NMR (CDCl 3 , 500MHz) δ3.03(q, 1H), 3.38(q, 1H), 3.56-3.60(m, 2H), 3.70(d, 1H), 4.44(d, 2H), 6.95(d, 1H), 7.00 -7.02(m, 2H), 7.11(t, 1H), 7.17-7.23(m, 6H), 7.26-7.33(m, 4H), 7.37(d, 1H); MS(ESI) m / z 406[M +Na] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com