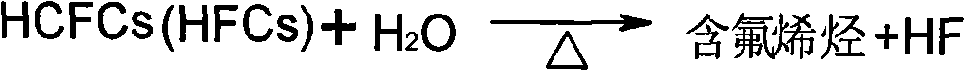Method for preparing fluorinated alkene by high temperature wet-cracking
A technology of fluorine-containing olefins and wet method, which is applied in the field of high-temperature wet cracking to prepare fluorine-containing olefins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Comparison of dehydrofluorination reaction and dry cracking reaction for HFC-236fa steam wet cracking.
[0029] The parameters of wet cracking: the temperature of the inner tube of the reactor is the reaction temperature, the reactant CF 3 CH 2 CF 3 The intake flow rate is 30ml / min, the water vapor flow rate is calculated based on the evaporation rate of 0.6g / min, and the reaction time is 0.04s. The experimental results are listed in Table 1, the main by-products are CO, CO2.
[0030] CF 3 CH 2 CF 3 → CF 3 CH=CF 2 +HF
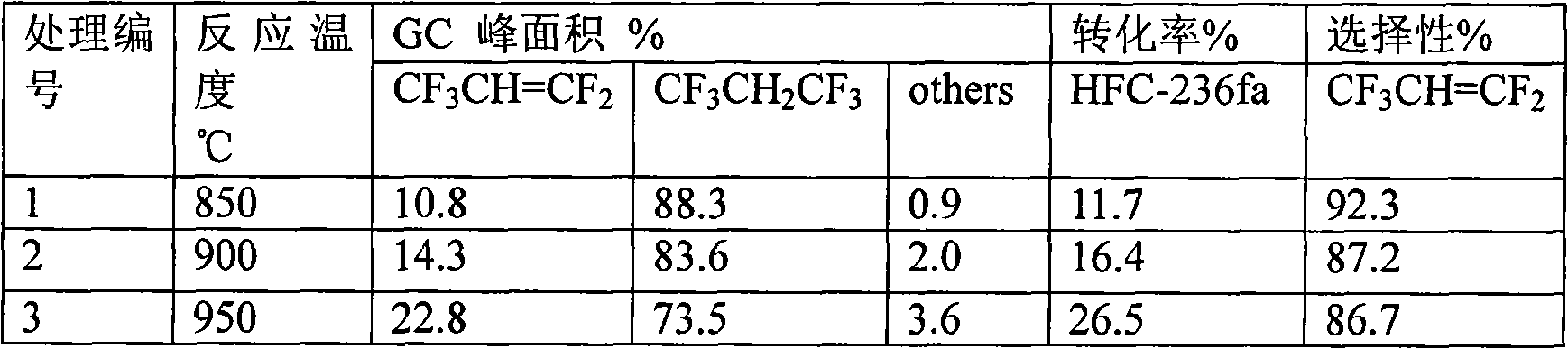
[0031] Table 1 Experimental results of wet cracking HFC-236fa
[0032]
[0033] Take the cracking dehydrofluorination reaction without adding water vapor or ammonia water vapor as a control:
[0034] The temperature of the inner tube of the reactor is the reaction temperature, the inlet flow rate of the reactant CF3CH2CF3 is 30ml / min, and the reaction time is 0.04s. The experimental results are listed in Table 2. The main by-produ...
Embodiment 2
[0039] Example 2 Influence of the amount of water vapor at 850°C on the dehydrofluorination reaction of wet cracking HFC-236fa
[0040] The reactor is the same as above, the temperature of the inner tube of the reactor is the reaction temperature, the inlet flow rate of the reactant CF3CH2CF3 is 30ml / min, the water vapor flow rate is calculated by evaporation per minute, and the reaction time is about 0.04s. The reaction conditions and corresponding experimental results are listed in Table 3. The main by-products are CO, CO2.
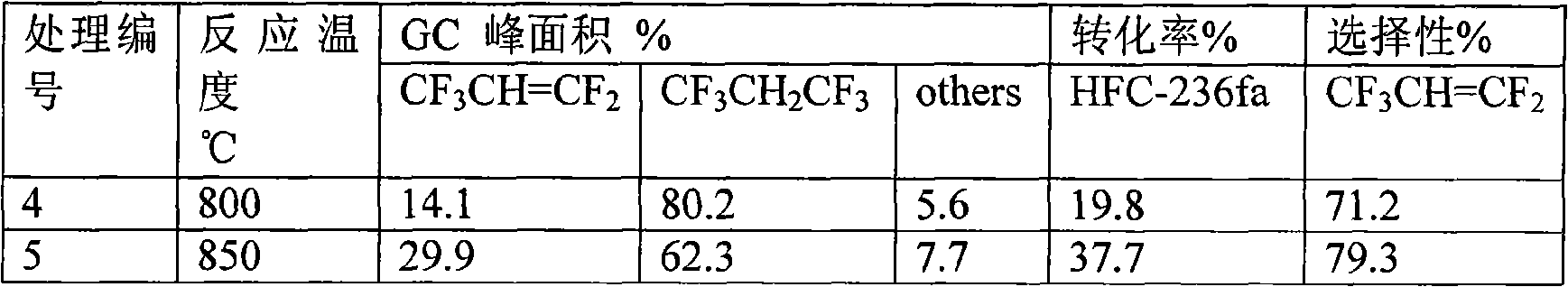
[0041] Table 3 Experimental results of steam wet cracking of HFC-236fa at 850℃
[0042]
[0043] The results show that within the selected water vapor flow rate range of the present invention, the conversion rate does not change substantially at 850°C, and the selectivity slightly increases with the increase of the water vapor amount.
Embodiment 3
[0044] Embodiment 3. The influence of reaction temperature on steam wet cracking HFC-245fa de-HF reaction
[0045] The reaction process is as follows:
[0046]
[0047] The temperature of the inner tube of the reactor is the reaction temperature, the inlet flow of the reactant CF3CH2CHF2 is 30ml / min, the water vapor flow is calculated based on the evaporation of 0.6g / min, and the reaction time is 0.04s. The reaction conditions and results are shown in Table 4, and the main by-products are CO and CO2.
[0048] Table 4 Effect of reaction temperature on experimental results of steam wet cracking of HFC-245fa
[0049]
[0050] The experimental results show that under the above conditions, as the temperature increases, the conversion rate of wet cracking HFC-245fa increases gradually, and the selectivity of trans and cis tetrafluoropropene decreases gradually. At 750-800°C, the conversion rate reaches more than 95%, and the by-products are about 0.2-1.1%. The obtained resul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com