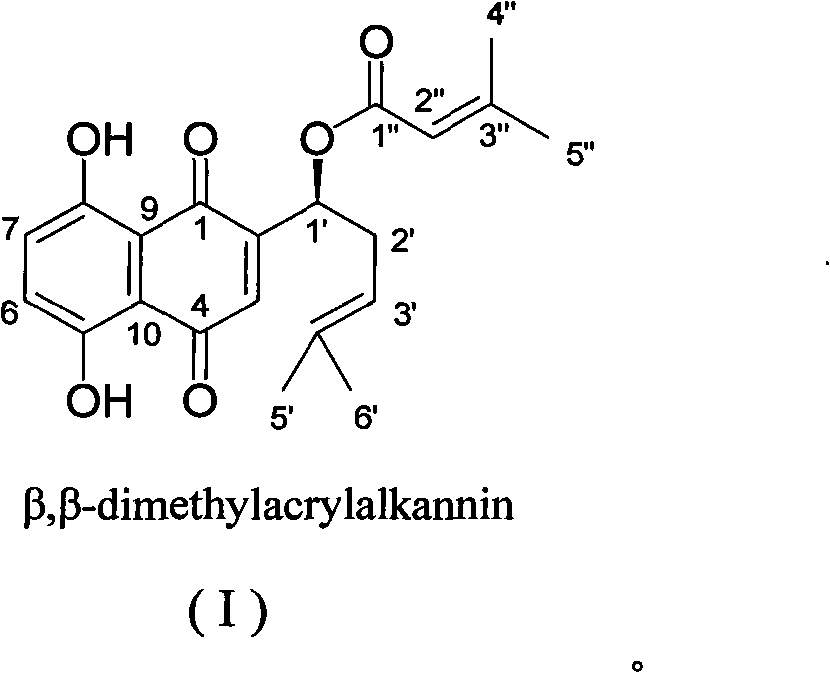
Beta,beta-dimethyl-acry-lalkannin and application in preparing medicines for inhibiting drug-resistant bacteria
A technology of drugs and bacteria, applied in the field of pharmacy, can solve the problems of treatment spread and epidemic difficulties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1 prepares compound AE02-7-2
[0038] 1.0 kg root of Arnebia euchroma plant, crushed, soaked in 95% ethanol below 45°C for extraction, combined extracts were concentrated under reduced pressure until all ethanol volatilized, and solid-phase extracted with chloroform. The remaining part was separated by silica gel and RP-18 forward and reverse phase column chromatography several times to obtain purple red solid compound AE02-7-2. Mix 2.0 g of the sample with 100-200 mesh silica gel, and evaporate the residual solvent. Take 250 grams of 200-300 mesh silica gel and put it into a glass column with a diameter of 2.0 cm, add the above-mentioned sample that has been mixed with silica gel and evaporated to dryness, and add mobile phase for separation. The mobile phase of silica gel column chromatography was chloroform-petroleum ether (1:1); the same fractions were combined and evaporated under reduced pressure to remove the solvent. Detect gained sample purity with...
Embodiment 2
[0041] Example 2 Compound AE02-7-2 Pharmacological activity test on SA1199B
[0042] Determine the minimum inhibitory concentration: Norfloxacin (norfloxacin) was purchased from Sigma Chemical Co. (Sigma Chemical Co.) Mueller-Hinton broth (Mueller-Hinton broth, MHB; Oxoid) adjusted to contain 20 mg of calcium ions and Magnesium ions. Bacterial cultures were formulated to 5 x 10 by comparison to McFarland standards 5 Inoculum at cfu / ml density. Norfloxacin and compound AE02-7-2 were dissolved in DMSO solvent and diluted in MHB to an initial concentration of 512 μg / ml. Use a Nunc type 96 microwell plate, add 125 microliters of MHB into wells 1-11; add 125 microliters of compound or norfloxacin (control drug) into well 1 and serially dilute, leaving well 11 without compound or Drugs served as bacterial growth controls. The final volume was added to well 12, which contained no broth as a sterile control. Finally, bacterial inoculum (125 microliters) was added to wells 1-11, r...
Embodiment 3
[0045] The pharmacological activity test of embodiment 3 compound AE02-7-2 to drug-resistant bacteria Xu212 and
[0046] Bacteria were cultured in the same manner as in Example 2, with norfloxacin as the positive control drug, and the experimental steps of Example 2 were repeated to detect the minimum inhibitory concentration of AE02-7-2 to drug-resistant strain Xu212. The strain Xu212 is a tetracycline-resistant Staphylococcus aureus strain with TetK tetracycline efflux protein (Richardson JF, Reith S. Journal of Hospital Infection, 1993, 25: 45-52.).
[0047] The results showed that the compound AE02-7-2 had an inhibitory effect on tetracycline-resistant Staphylococcus aureus strain Xu212 with TetK tetracycline efflux protein, and the minimum inhibitory concentration was 4 μg / ml (10.8 μM), which was comparable to that of the positive control drug norflu The antibacterial activity of Floxacin is comparable, 4 micrograms / ml (12.5μM).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com