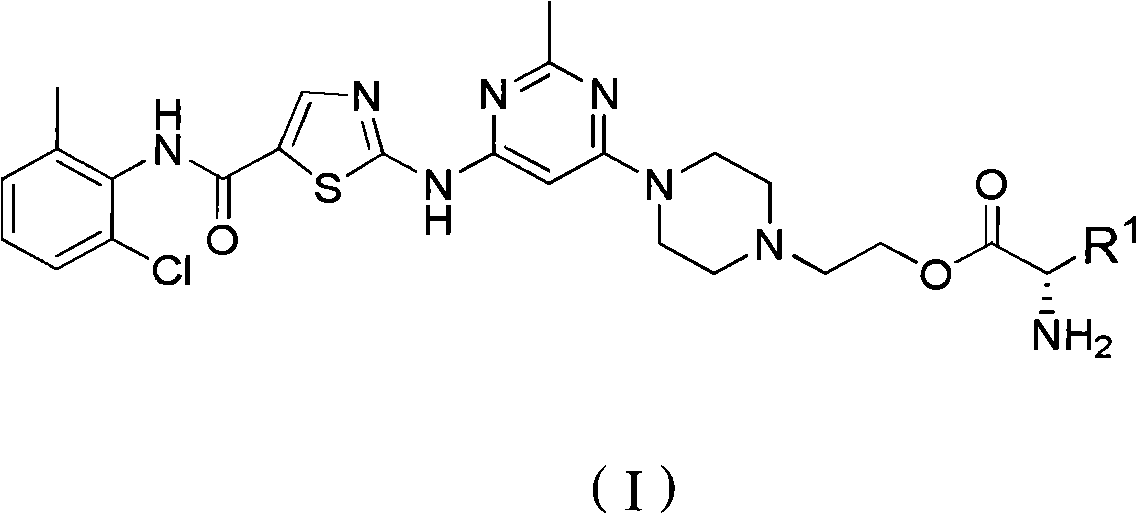
Anti-tumor compound preparation method
A technology of compounds and alkaline conditions, applied in the fields of anti-tumor drugs, organic chemistry, drug combinations, etc., can solve the problems of high side effects, large differences in individual treatment of patients, and low bioavailability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
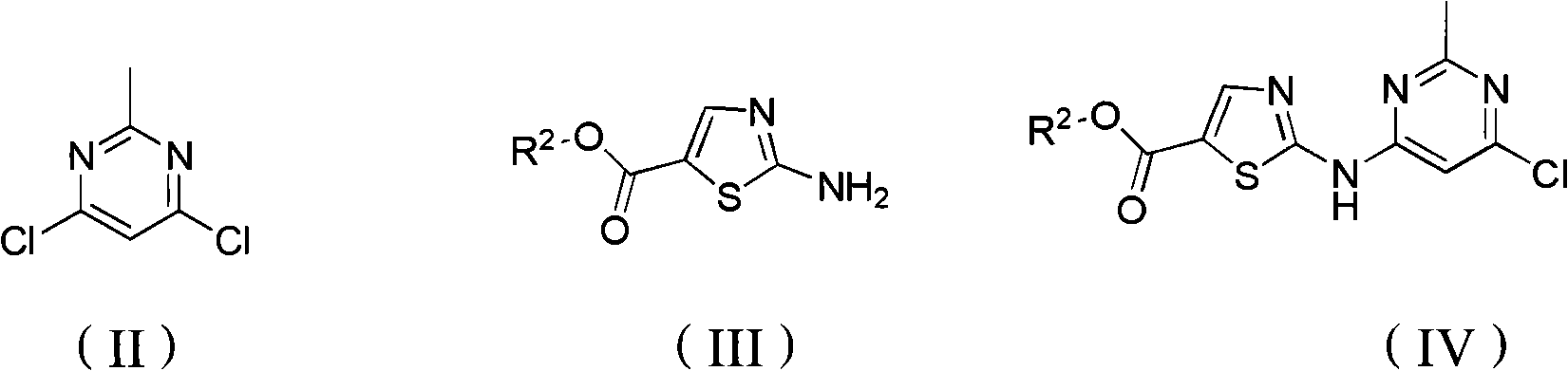
[0075] Example 1: Preparation of ethyl 2-(6-chloro-2-methylpyrimidine-4-amino)thiazole-5-carboxylate (IV)
[0076] Add 20ml DMF to a 100ml three-necked flask, stir magnetically, mix 2g ethyl 2-aminothiazole-5-carboxylate, 7.5g Cs 2 CO 3 (2eq), 2.85g (1.5eq) of 4,6-dichloro-2-methylpyrimidine was dissolved in 6 ml of DMF, and slowly dropped into the reaction flask. After the dropwise addition was completed, the ice-water bath was removed, and the mixture was naturally raised to room temperature (25° C.). After 24 hours of reaction, the reaction of ethyl 2-amino-thiazole-5-carboxylate was complete. The system was poured into 250ml of ice water, stirred, a solid precipitated out, filtered with suction, and the filter cake was washed twice with water and ethyl acetate (EA) successively, and dried to obtain 2.7g of a light white solid with a yield of 78%. The compound is C 11 h 11 ClN 4 o 2 S, the calculated value of MS-ESI (m / z) is 298.0; the measured value is 297.2 (100, M ...
Embodiment 2
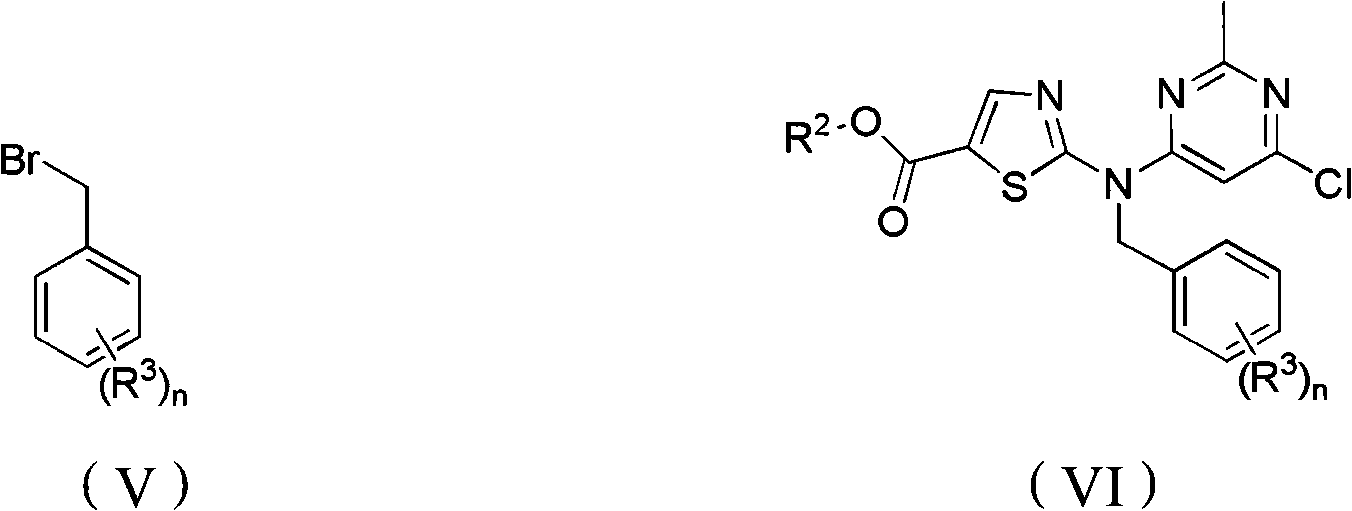
[0077] Example 2: Preparation of ethyl 2-((6-chloro-2-methylpyrimidin-4-yl)(4-methoxybenzyl)amino)thiazole-5-carboxylate (VI)
[0078] Add 150ml DMF, 20g (IV) prepared in Example 1, 18.76g K 2 CO 3 (2eq), there are still some raw materials (IV) undissolved. 20.1g p-methoxybenzyl bromide (2eq) was dissolved in 50ml DMF, dropped into the reaction flask, the temperature rose to 5°C, after the dropwise addition was completed, the ice bath was removed, and the temperature was naturally raised to room temperature. After 18 hours of reaction, the raw material ( IV) The reaction is complete. The reaction solution was poured into 300ml of ice water, filtered with suction, washed with water and EA three times in turn, and dried to obtain 25g of white solid with a yield of 89.3%. The compound is C 19 h 19 ClN 4 o 3 S, the calculated value of MS-ESI (m / z) is 418.1; the measured value is 419.1 (100, MH + ).
Embodiment 3
[0079] Example 3: Preparation of 2-((6-chloro-2-methylpyrimidin-4-yl)(4-methoxybenzyl)amino)thiazole-5-carboxylic acid (VII)
[0080] Add 15ml of water, 90ml of THF, and 90ml of methanol into a 250ml three-necked flask, cool down to 0°C with an ice-water bath, add 8.38g of (VI) prepared in Example 2, and part of (VI) is not dissolved. Dissolve 8.0g (10eq) of sodium hydroxide in 15ml of ice water, slowly drop it into the reaction flask, remove the ice bath after the dropwise addition, the insoluble matter gradually dissolves, and after 5 hours of reaction (VI), the reaction is complete, and the organic solvent is evaporated , adding 100ml of water, filtering, washing with water twice, and drying to obtain 5.6g of white solid with a yield of 71.8%. The compound is C 17 h 15 ClN 4 o 3 S, the calculated value of MS-ESI (m / z) is 390.1; the measured value is 389.3 (M - ) and 345.3 (M - ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com