Rapid material cooling and waste acid recycling process after chlorobenzene thermal insulation nitration
An adiabatic nitration and rapid cooling technology, applied in the preparation of nitro compounds, the preparation of organic compounds, organic chemistry, etc., can solve problems such as short process flow, avoid side reactions and save energy consumption.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
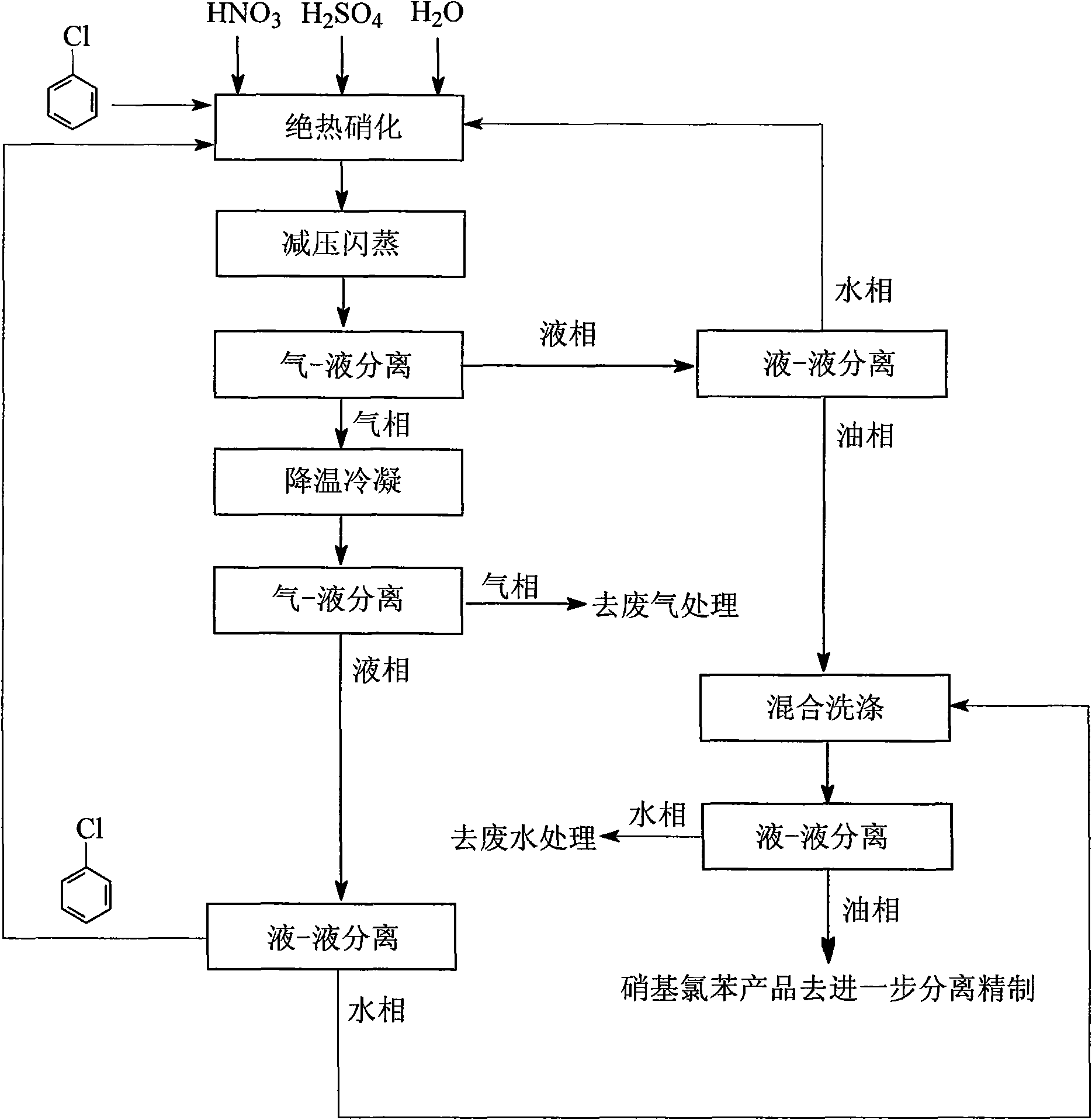
[0026] The main process equipment is: adiabatic nitrification reactor, reduced pressure flash evaporation equipment, cooling and condensation equipment, gas-liquid separation equipment and liquid-liquid separation equipment, etc.
[0027] Such as figure 1 As shown, a process for rapid cooling of materials after adiabatic nitration of chlorobenzene and recycling of waste acid, the process steps are as follows:
[0028] (1) Adiabatic nitrification: In the adiabatic nitrification static mixing reactor, the mixed acid of the nitration reaction is made by mixing industrial grade mass fraction of 98% sulfuric acid, 65% nitric acid and water in a certain proportion, and the dehydration value of the mixed acid is 4.0, chlorine The molar ratio of benzene to nitric acid is 1.05:1.0. Mix chlorobenzene and mixed acid to cause nitration reaction. The temperature of the materials after the nitration reaction is about 120°C, and the reacted materials enter the next step;
[0029] (2) Reduced pre...
Embodiment 2
[0038] The main process equipment is: adiabatic nitrification reactor, reduced pressure flash evaporation equipment, cooling and condensation equipment, gas-liquid separation equipment and liquid-liquid separation equipment, etc.
[0039] Such as figure 1 As shown, a process for rapid cooling of materials after adiabatic nitration of chlorobenzene and recycling of waste acid, the process steps are as follows:
[0040] (1) Adiabatic nitrification: In the SV type adiabatic nitrification static mixing reactor, the mixed acid of the nitration reaction is mixed with industrial grade mass fraction of 98% sulfuric acid, 65% nitric acid and waste acid in a certain proportion. The dehydration value of the mixed acid is 3.0, the molar ratio of chlorobenzene to nitric acid is 1.1:1.0, mixing chlorobenzene and mixed acid to cause a nitration reaction, the temperature of the material after the nitration reaction is about 140°C, and the reacted material enters the next step;
[0041] (2) Flash ...
Embodiment 3
[0050] The main process equipment is: adiabatic nitrification static mixing reactor, reduced pressure flash evaporation equipment, cooling and condensation equipment, gas-liquid separation equipment and liquid-liquid separation equipment, etc.
[0051] Such as figure 1 As shown, a process for rapid cooling of materials after adiabatic nitration of chlorobenzene and recycling of waste acid, the process steps are as follows:
[0052] (1) Adiabatic nitrification: In the SV type adiabatic nitrification static mixing reactor, the mixed acid of the nitration reaction is mixed with industrial grade mass fraction of 98% sulfuric acid, 65% nitric acid and waste acid in a certain proportion. The dehydration value of the mixed acid is 2.0, the molar ratio of chlorobenzene to nitric acid is 1.1:1.0, mixing chlorobenzene and mixed acid to produce a nitration reaction, the temperature of the material after the nitration reaction is about 160°C, and the reacted material enters the next step;
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com