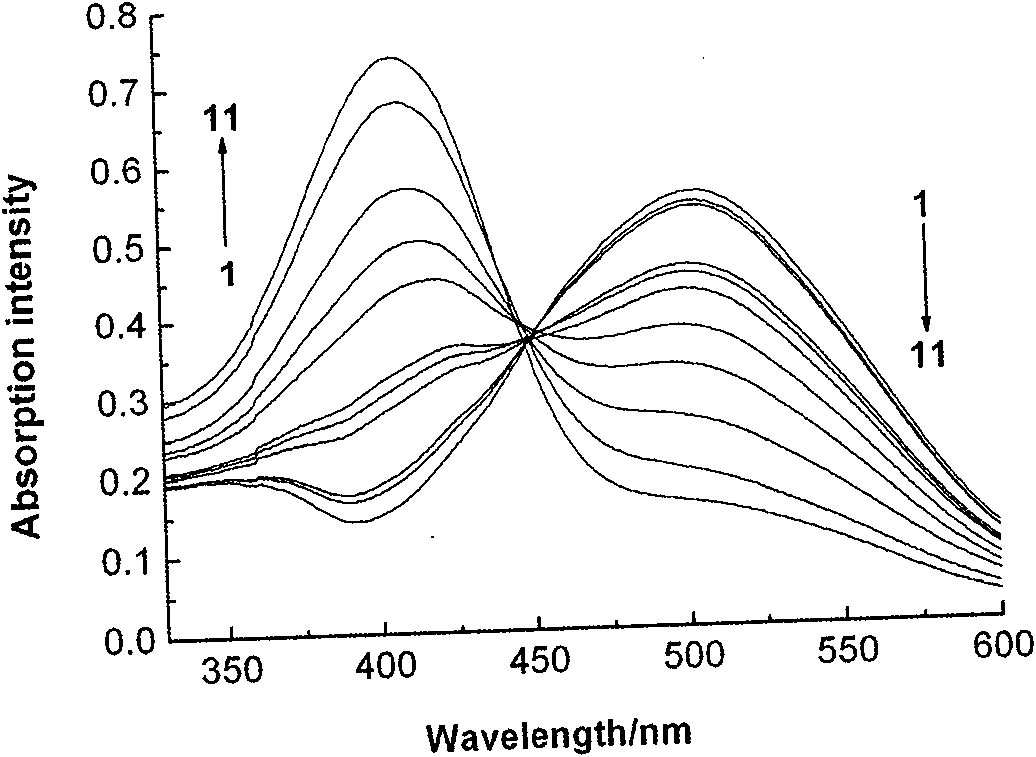
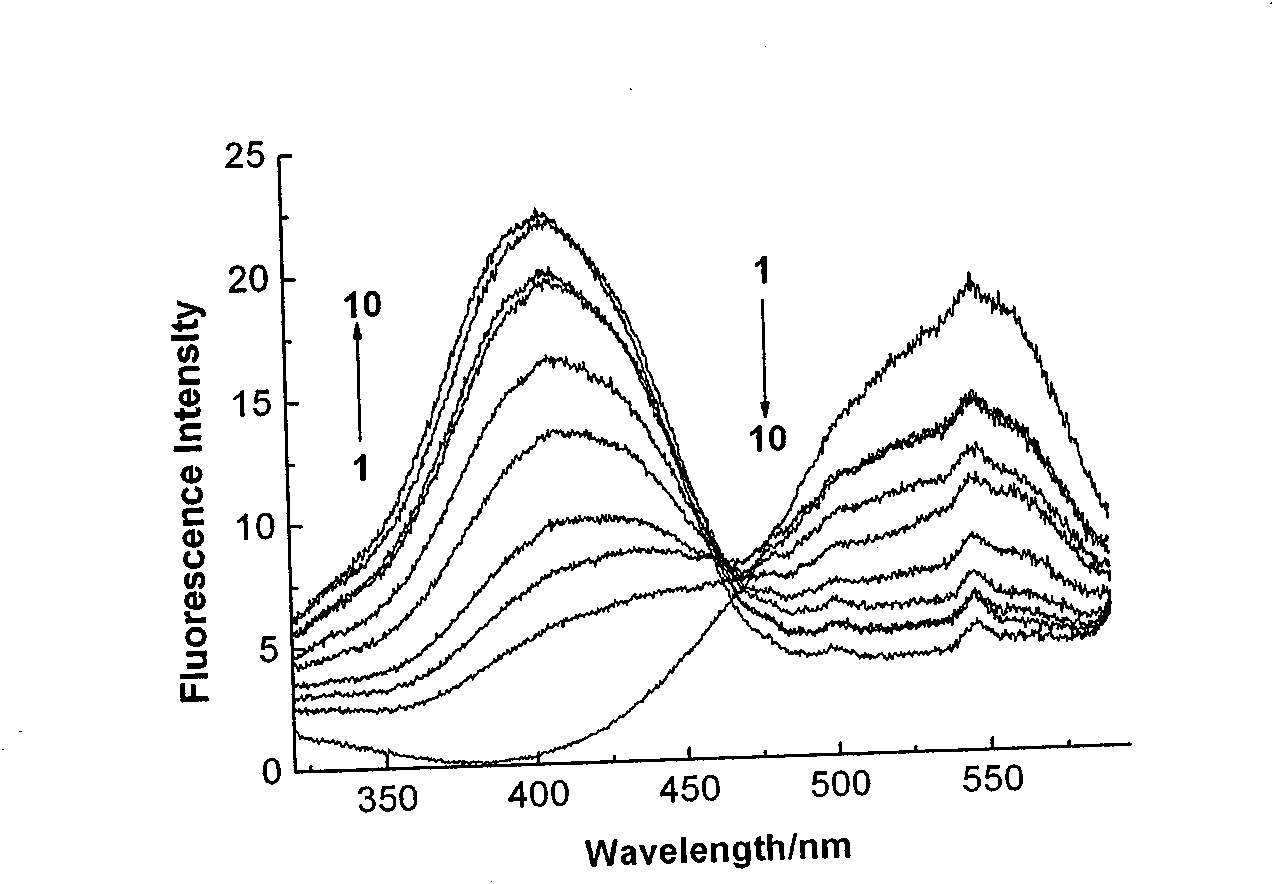
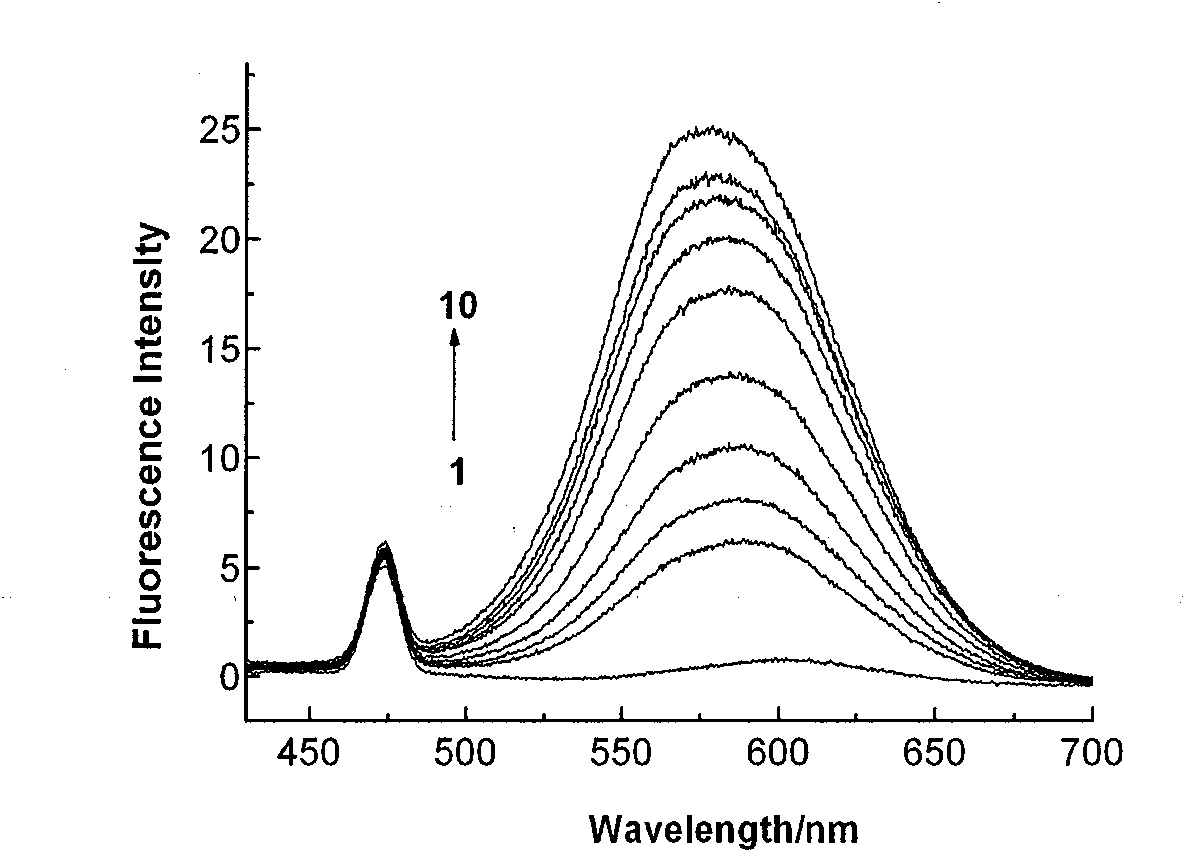
Subcellular area targeting, long-wavelength and dual-wavelength ratio method Ca<2+> fluorescent probe and synthesis method thereof
A technology of fluorescent probes and synthesis methods, applied in the field of biochemical sensing research, can solve the problems of non-targeting, difficult dual-wavelength ratiometric measurement, and inability to use subcellular measurement, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) Weigh 29.42g (50mmol) of 1,2-bis(2-aminophenoxy)ethane-N,N,N',N'-tetraethyl acetate into a 250mL conical flask, add 150mL to dry Dimethylformamide, stirring to dissolve 1,2-bis(2-aminophenoxy)ethane-N,N,N',N'-tetraethyl acetate. Cool to -2℃, add POCl dropwise 3 18.14mL (200mmol), need 2h, then stir at room temperature for about 40h. After the reaction, the reaction mixture was poured into 500 mL of deionized water at 0°C under stirring, and there was precipitation. Adjust the suspension to neutral with 5% KOH solution, filter with suction, and redissolve the resulting crude product in ethyl acetate. In the ester, wash twice with deionized water and saturated NaCl solution respectively. Anhydrous Na for organic phase 2 SO 4 After drying, filtering, and evaporating ethyl acetate under reduced pressure on a rotary evaporator to obtain a brown oil, the pure product 5,5'-dialdehyde-BAPTA-tetraethyl ester is obtained by column chromatography. The reaction formula of this...
Embodiment 2
[0037] (1) Weigh 14.71g (25mmol) of 1,2-bis(2-aminophenoxy)ethane-N,N,N',N'-tetraethyl acetate into a 250mL conical flask, add 150mL to dry Dimethylformamide, stirring to dissolve 1,2-bis(2-aminophenoxy)ethane-N,N,N',N'-tetraethyl acetate. Cool to -2℃, add POCl dropwise 3 18.14mL (200mmol), need 2h, then stir at room temperature for about 40h. After the reaction, the reaction mixture was poured into 500 mL of deionized water at 0°C under stirring, and there was precipitation. Adjust the suspension to neutral with 5% KOH solution, filter with suction, and redissolve the resulting crude product in ethyl acetate. In the ester, wash twice with deionized water and saturated NaCl solution respectively. Anhydrous Na for organic phase 2 SO 4 After drying, filtering, and evaporating ethyl acetate under reduced pressure on a rotary evaporator to obtain a brown oil, the pure product 5,5'-dialdehyde-BAPTA-tetraethyl ester is obtained by column chromatography. The reaction formula of this...
Embodiment 3
[0044] (1) Weigh 11.77g (20mmol) of 1,2-bis(2-aminophenoxy)ethane-N,N,N',N'-tetraethyl acetate into a 250mL conical flask, add 70mL to dry Dimethylformamide, stirring to dissolve 1,2-bis(2-aminophenoxy)ethane-N,N,N',N'-tetraethyl acetate. Cool to -2℃, add POCl dropwise 3 9.07mL (100mmol), need 2h, then stir at room temperature for 40h. After the reaction, the reaction mixture was poured into 250 mL of deionized water at 0°C under stirring, and precipitation was precipitated. Adjust the suspension to neutral with 5% KOH solution, filter with suction, and redissolve the resulting crude product in ethyl acetate. In, and wash with deionized water and saturated NaCl solution twice. Anhydrous Na for organic phase 2 SO 4 After drying, filtering, and evaporating ethyl acetate under reduced pressure on a rotary evaporator to obtain a brown oil, the pure product 5,5'-dialdehyde-BAPTA-tetraethyl ester is obtained by column chromatography. The reaction formula of this step is as follows:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com