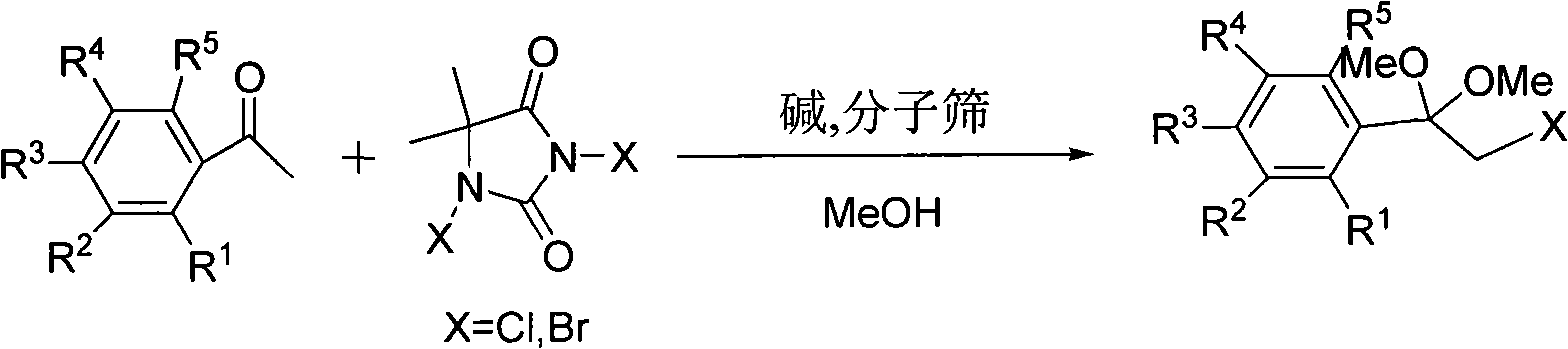
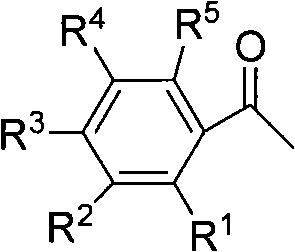
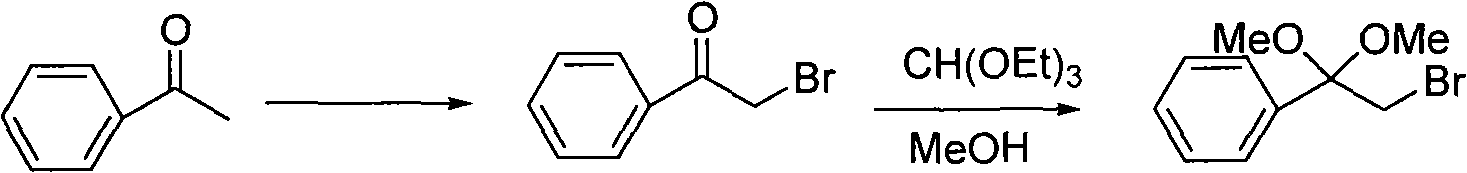
One-step method for preparing alpha-halo acetophenone dimethyl ketal compounds
A technology of halogenated acetophenone dimethyl ketal and compound, which is applied in the field of preparation of fine chemical products, and can solve the problems of difficult product purification and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Preparation of α-chloro-p-methylacetophenone dimethyl ketal from p-methylacetophenone
[0034] Add 15mmol dichlorohydantoin, 10mmol p-toluophenone, 35ml anhydrous methanol, 15g molecular sieves, 5mmol hexahydropyridine into a 50ml three-necked flask, and heat to reflux for 12h. Filter out molecular sieves, then distill methanol off, then dissolve with 50ml methyl tert-butyl ether or dichloromethane and 40ml water, separate the liquid with a separatory funnel, wash twice with saturated saline, dry over anhydrous sodium sulfate, filter, and distill off the solvent The product α-chloro-p-methylacetophenone dimethyl ketal was obtained with a yield of 70%. Product IR(cm -1 ): 1118, 1060, 1040, 1001.
Embodiment 2
[0036] Preparation of α-chloroacetophenone dimethyl ketal from acetophenone
[0037] Add 15mmol of dichlorohydantoin, 10mmol of acetophenone, 35ml of anhydrous methanol, 10g of molecular sieves, and 2.5mmol of triethylamine into a 50ml three-necked flask, and heat to reflux for 10h. Filter out molecular sieves, then distill methanol off, then dissolve with 50ml methyl tert-butyl ether or dichloromethane and 40ml water, separate the liquid with a separatory funnel, wash twice with saturated saline, dry over anhydrous sodium sulfate, filter, and distill off the solvent The product α-chloroacetophenone dimethyl ketal was obtained with a yield of 80%. Product IR(cm -1 ): 1120, 1070, 1045, 1006.
Embodiment 3
[0039] Preparation of α-chloro-p-bromoacetophenone dimethyl ketal from p-bromoethanone
[0040] Add 15mmol dichlorohydantoin, 10mmol p-bromoacetophenone, 35ml anhydrous methanol, 15g molecular sieves, 3mmol n-butylamine into a 50ml three-necked flask, and react at room temperature for 24h. Filter out molecular sieves, then distill methanol off, then dissolve with 50ml methyl tert-butyl ether or dichloromethane and 40ml water, separate the liquid with a separatory funnel, wash twice with saturated saline, dry over anhydrous sodium sulfate, filter, and distill off the solvent The product α-chloro-p-bromoacetophenone dimethyl ketal was obtained with a yield of 90%. Product IR(cm -1 ): 1124, 1073, 1051, 1011.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com