Lentinan lyophilized power injection and preparation method thereof
A technology of freeze-dried powder injection and lentinan, applied in the field of lentinan freeze-dried powder injection and its preparation, can solve the problems of unhelpful treatment, adverse side effects, adverse reactions, etc., reduce adverse reactions, reduce toxic and side effects, and improve safety sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
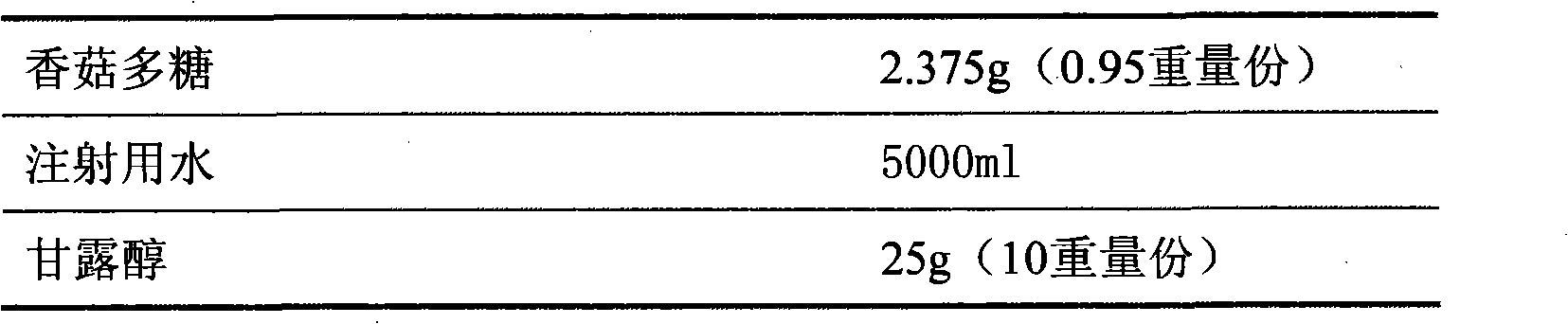
[0020] Preparation prescription:
[0021]
[0022] The preparation method of lentinan freeze-dried powder injection is characterized in that the specific steps are:
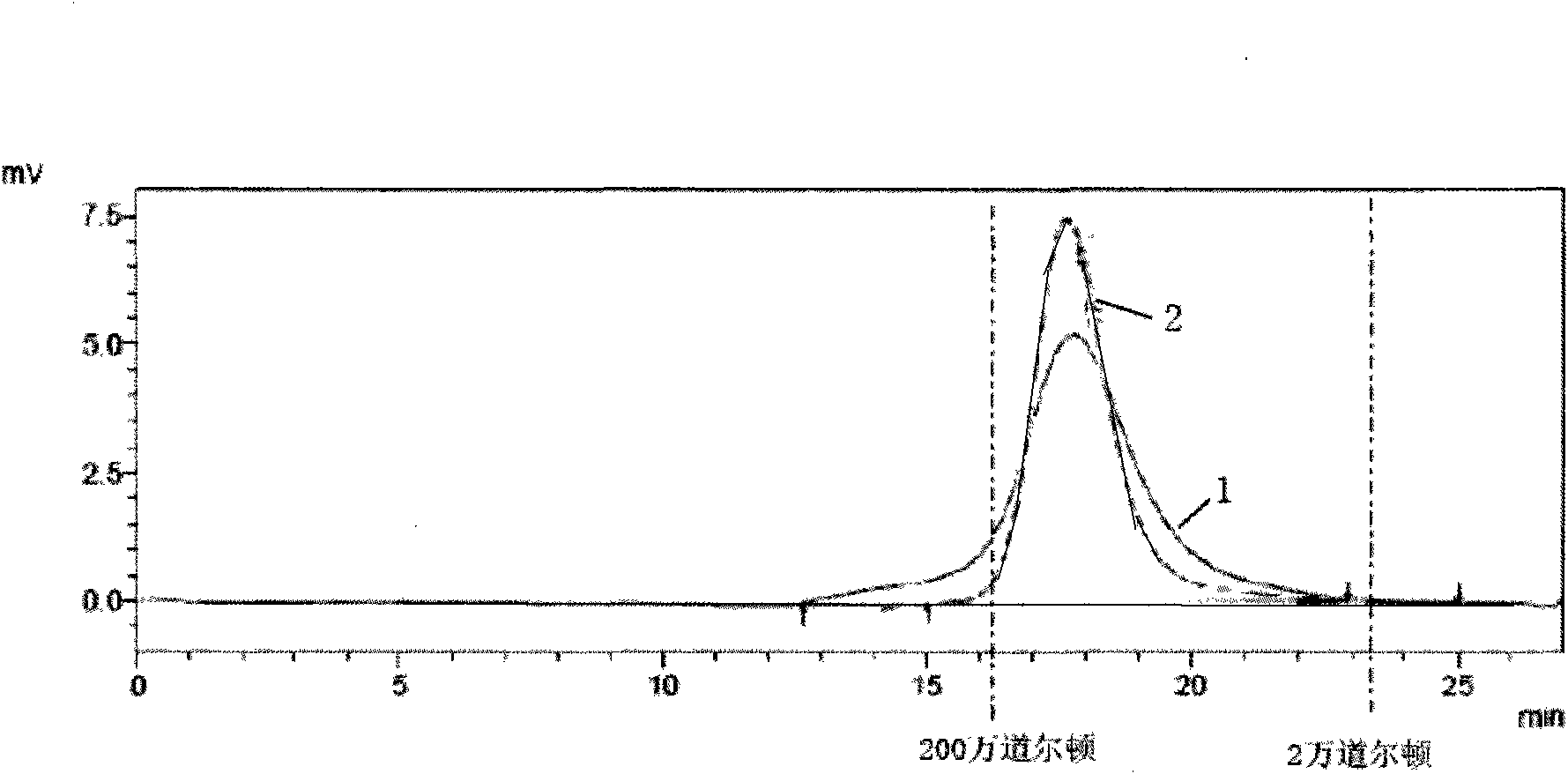
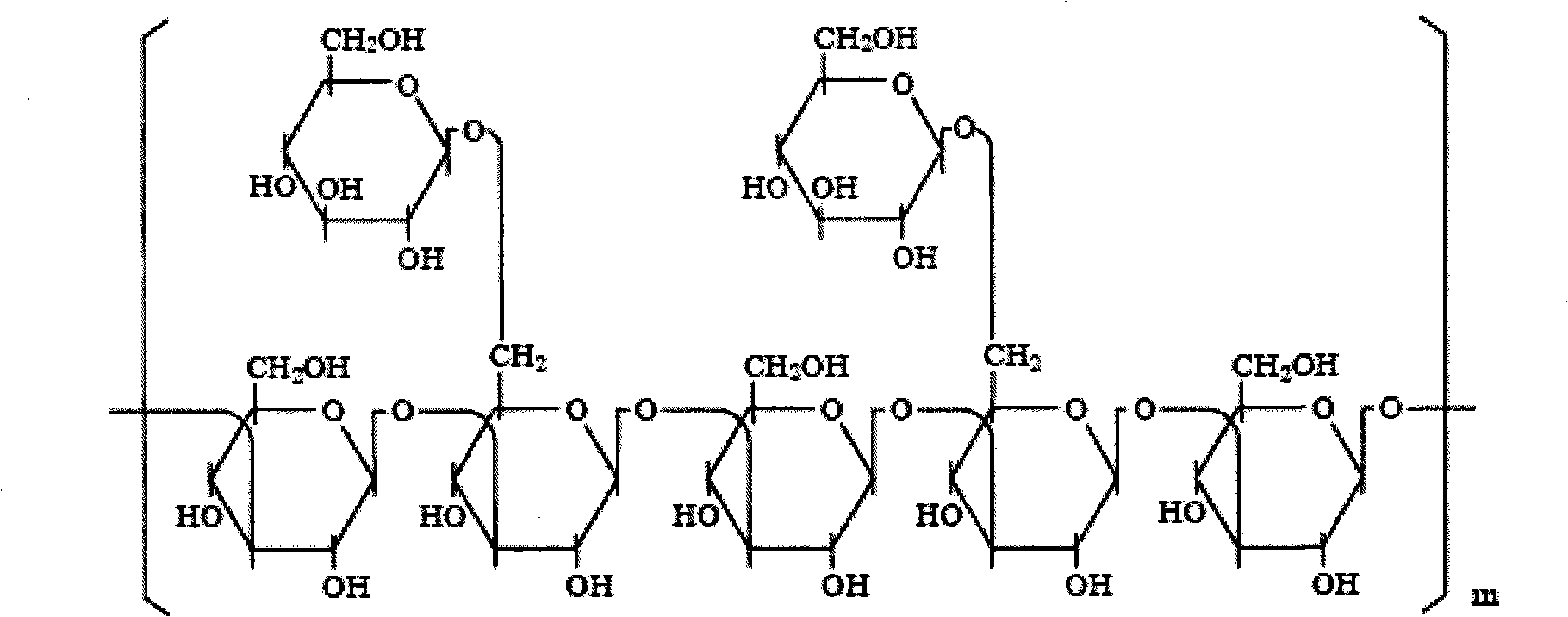
[0023] Step 1: Dissolve the lentinan that has met the national standard with water into a 0.2g / L lentinan solution. At 5°C, put the solution into a dialysis bag with a molecular weight cut-off of 10,000 Daltons and dialyze against water. Change the water once every 4 hours, and perform dialysis for 36 hours; filter the solution obtained by dialysis twice with a microfiltration membrane with a pore size of 0.14 μm at room temperature, and then ultrafiltrate the obtained filtrate with an ultrafiltration membrane with a molecular weight cut-off of 500,000 Daltons. The pressure difference of the ultrafiltration feed is set to 0.18MPa, the filtrate obtained by the ultrafiltration is concentrated under reduced pressure to 1 / 8 of the original volume, and freeze-dried to obtain a compound with a weight average molecul...
Embodiment 2
[0028] Preparation prescription:
[0029]
[0030] The preparation method of lentinan freeze-dried powder injection is characterized in that the specific steps are:
[0031] The first step: Dissolve the lentinan that has met the national standard with water into a 0.3g / L lentinan solution. At 20°C, put the solution into a dialysis bag with a molecular weight cut-off of 10,000 Daltons and dialyze against water. Change the water once every 6 hours, and perform dialysis for 48 hours; filter the solution obtained by dialysis twice at room temperature with a microfiltration membrane with a pore size of 0.2 μm, and then ultrafiltrate the obtained filtrate with an ultrafiltration membrane with a molecular weight cut-off of 1 million Daltons. The pressure difference of the ultrafiltration feed is set to 0.15MPa, the filtrate obtained by the ultrafiltration is concentrated under reduced pressure to 1 / 6 of the original volume, and then freeze-dried to obtain a Lentinan with a compon...
Embodiment 3
[0036] Preparation prescription:
[0037]
[0038] The preparation method of lentinan freeze-dried powder injection is characterized in that the specific steps are:
[0039] The first step: Dissolve the lentinan that has met the national standard with water into a 0.3g / L lentinan solution. At 20°C, put the solution into a dialysis bag with a molecular weight cut-off of 10,000 Daltons and dialyze against water. Change the water once every 6 hours, and perform dialysis for 48 hours; filter the solution obtained by dialysis twice at room temperature with a microfiltration membrane with a pore size of 0.2 μm, and then ultrafiltrate the obtained filtrate with an ultrafiltration membrane with a molecular weight cut-off of 1 million Daltons. The pressure difference of the ultrafiltration feed is set to 0.16MPa, the filtrate obtained by the ultrafiltration is concentrated under reduced pressure to 1 / 6 of the original volume, and then freeze-dried to obtain the Lentinan with a comp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com