Method for producing positive electrode material for secondary battery
A secondary battery and manufacturing method technology, applied in the direction of secondary batteries, battery electrodes, circuits, etc., can solve the problem of difficulty in controlling the crystallinity, particle size and particle size distribution of products, not necessarily suitable as positive electrode materials, and deterioration of charge and discharge characteristics, etc. problems, to achieve excellent charge and discharge characteristics, high occlusion and release efficiency, and improved stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
In addition to changing the silicon source to phenyl silicone (C 6 h 5 SiO 1.5 ) n Except, the rest were carried out under the same conditions as in Comparative Example 1. 2 FeSiO 4 Synthesis.
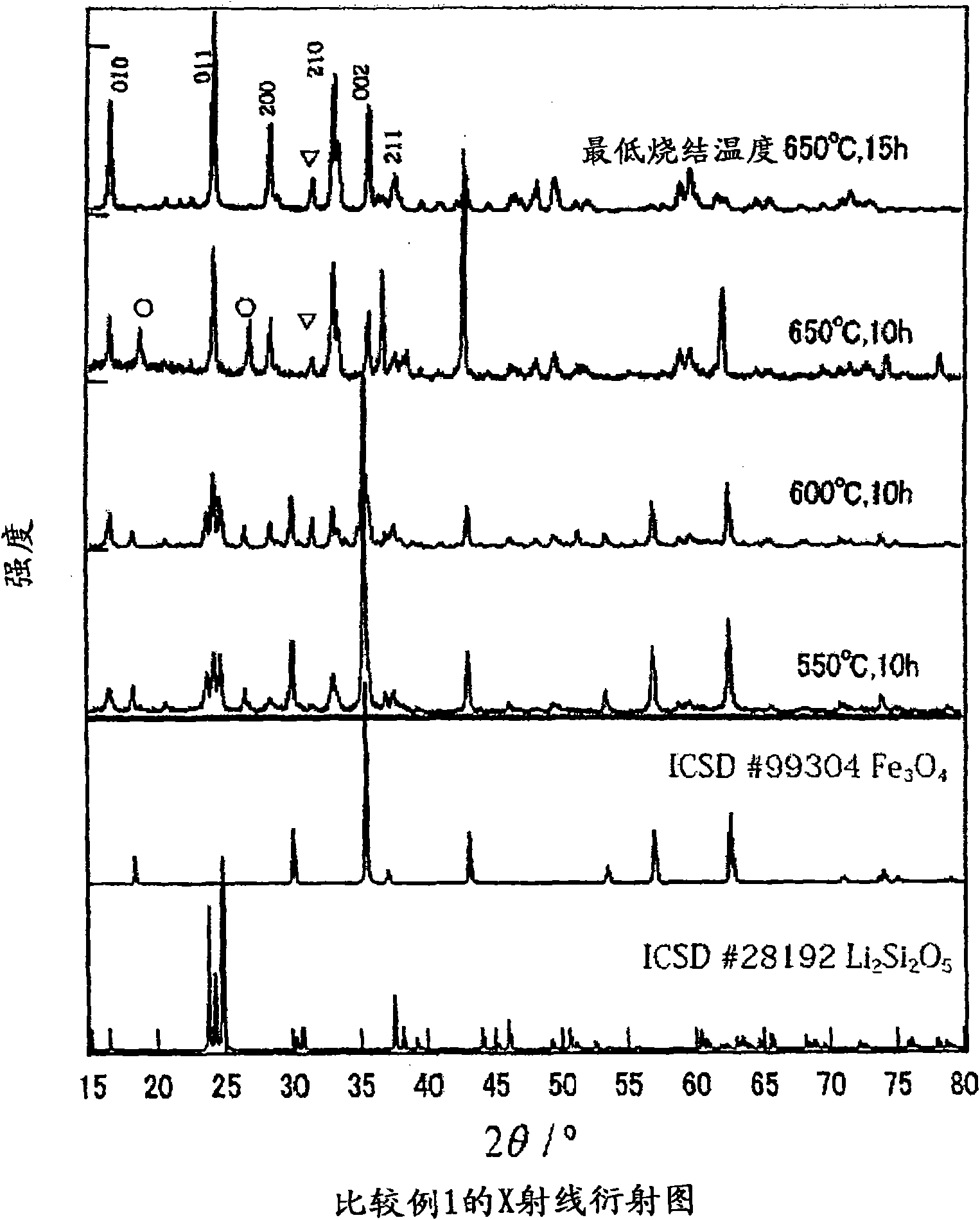
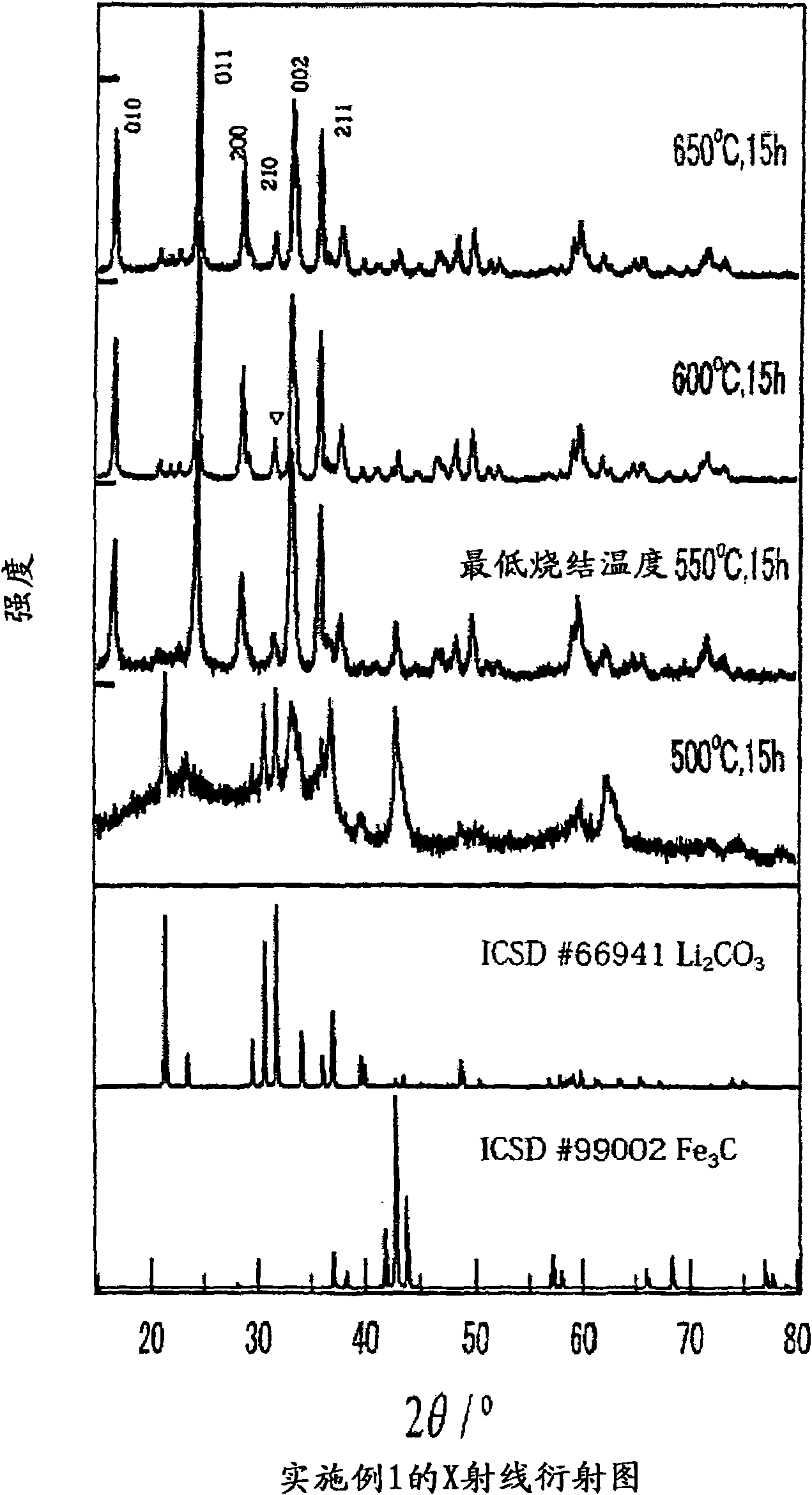
[0083] X-ray diffraction experiment
X-ray diffraction experiments were performed on the samples obtained in Comparative Example 1 and Example 1, respectively. Specifically, using CoKα rays monochromatized by a color filter, the tube voltage and tube current were set to 35kV and 40mA, respectively, and the measurement angle was set to 15°figure 1 , the X-ray diffraction pattern of Example 1 is shown in figure 2 .
[0084] From figure 1 and figure 2 It can be seen that in Comparative Example 1, when operating at a temperature below 650°C, there are many impurities remaining, and no single-phase Li 2 FeSiO 4 , compared with this, in Example 1, the single-phase Li 2 FeSiO 4 . Thus, by using (C 6 h 5 SiO 1.5 ) n As a raw material, the synthesis temperature can be lowered...
Embodiment 2
In addition to changing the lithium source to Li2 CO 3 , Change the silicon source to phenyl silicone resin (C 6 h 5 SiO 1.5 ) n Except, the rest were carried out under the same conditions as in Comparative Example 2. 2 MnSiO 4 Synthesis.
[0094] X-ray diffraction experiment
X-ray diffraction experiments were performed on the samples obtained in Comparative Example 2 and Example 2, respectively. Specifically, using CuKα rays monochromated by a color filter, the tube voltage and tube current were set to 50kV and 180mA, respectively, and the measurement angle was set to 15° Figure 5 . Figure 5 (a) in represents comparative example 2, Figure 5 (b) in (b) shows Example 2.
[0095] In Comparative Example 2, when operating at a temperature below 750°C, many impurities remained, and no single-phase Li 2 FeSiO 4 , by contrast, in Example 2, operating at 600 °C, an essentially single-phase Li 2 FeSiO 4 . Thus, by using (C 6 h 5 SiO 1.5 ) n As a raw material, the sy...
Embodiment 3
In addition to changing the silicon source to phenyl silicone (C 6 h 5 SiO 1.5 ) n The rest were synthesized under the same conditions as in Comparative Example 3.
[0105] X-ray diffraction experiment
X-ray diffraction experiments were performed on the samples obtained in Comparative Example 3 and Example 3, respectively. Specifically, using CuKα rays monochromated by a color filter, the tube voltage and tube current were set to 35kV and 40mA, respectively, and the measurement angle was set to 15° Figure 8 and Figure 9 . Figure 8 represents Comparative Example 3, Figure 9 Example 3 is shown.
[0106] In Comparative Example 3, operating at a temperature below 800°C, many impurities remained, and no single-phase Li 2 CoSiO 4 , by contrast, in Example 3, operating at 700 °C, an essentially single-phase Li 2 CoSiO 4 . Thus, by using (C 6 h 5 SiO 1.5 ) n As a raw material, the synthesis temperature can be lowered by about 100°C.
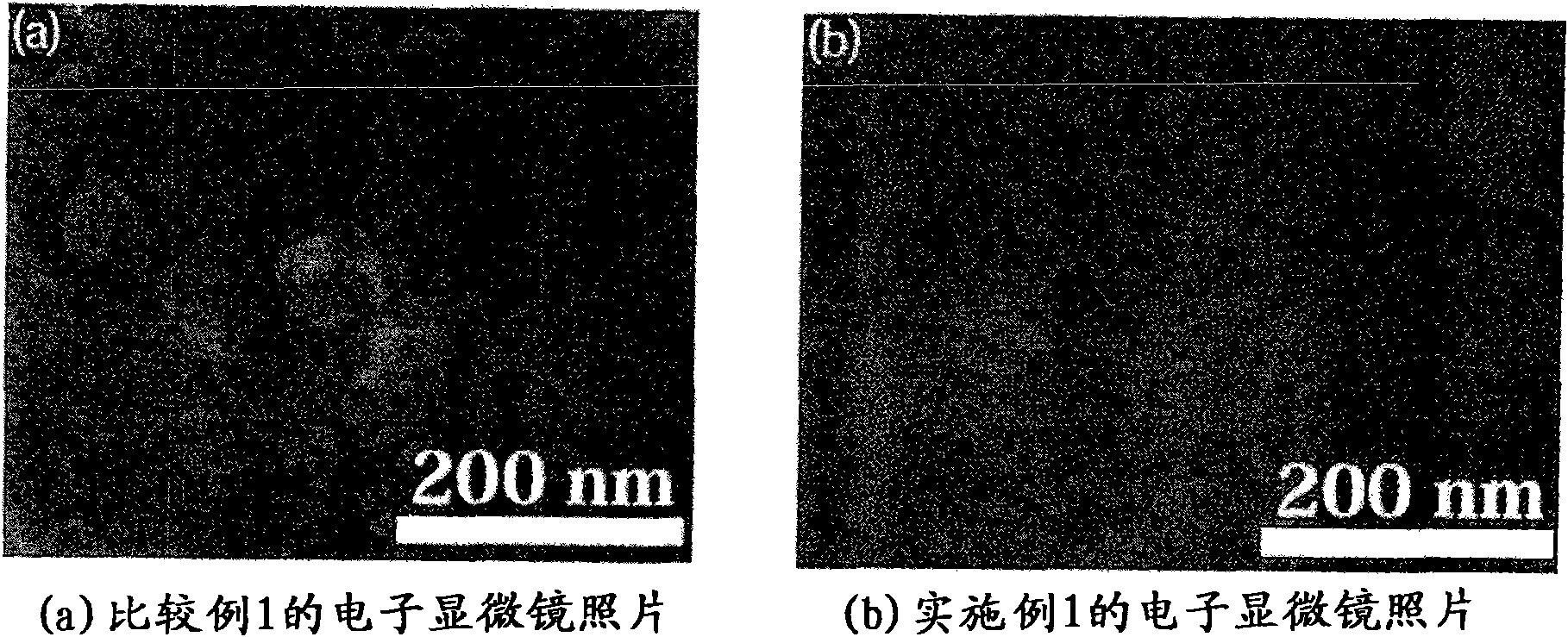
[0107] Electron microscope obs...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com