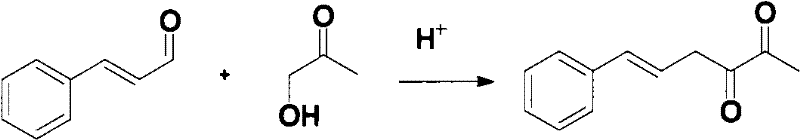Novel flavor compound and preparation method, application and composition thereof
A technology of compounds and compositions, applied in the fields of diketone compounds, uses and compositions thereof, and preparation methods thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
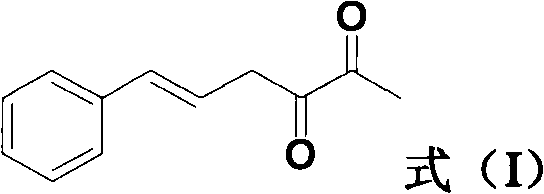
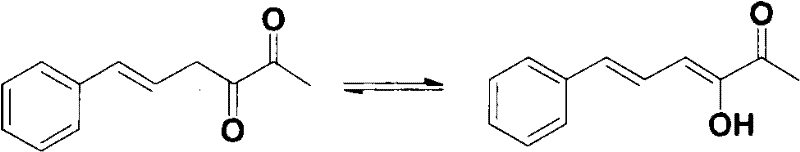
[0026] Example 1: Synthesis of 3-hydroxyl-6-phenyl-3,5-hexadien-2-one
[0027] Mix 100g of cinnamaldehyde and 286g of hydroxyacetone at room temperature to obtain a mixture of cinnamaldehyde and hydroxyacetone; add 63g of hydrochloric acid with a concentration of 15% into the reaction flask, heat up to 50°C, and then add the above-mentioned cinnamon dropwise at a constant speed within 2 hours while stirring Mixture of aldehyde and hydroxyacetone, continue to keep for 2 hours after dripping;
[0028] Then 110 g of water was added to the reaction liquid, and the reaction was continued for 2 h after the temperature was raised to 82° C. After the reaction, the product was obtained by filtration while hot, and 39.4 g of white crystals were obtained by recrystallization with ethyl acetate. After testing, its melting point was 127.8-128.1°C; the yield was 27.7%.
[0029] 1 H NMR (CDCl 3 , 400MHz): d 2.45(s, 3H), 6.39(d, J=11.2Hz, 1H), 6.84(d, J=12Hz, 1H), 6.86(s, 1H), 7.28-7.34(m,...
Embodiment 2
[0032] Example 2: Olfactory evaluation of pure 3-hydroxy-6-phenyl-3,5-hexadien-2-one
[0033] The olfactory evaluation of pure 3-hydroxy-6-phenyl-3,5-hexadien-2-one was carried out by a panel of several professionals. The compound has a pure and herbal aroma with notes of tobacco and beans aroma.
Embodiment 3
[0034] Example 3: Olfactory evaluation of 3-hydroxy-6-phenyl-3,5-hexadien-2-one in compositions
[0035] components Test group 1 (% by weight) Test group 2 (% by weight) Test group 3 (% by weight) ethanol 5% 2% 2.5%
[0036] squalane 50% 40% 60% Glyceryl triethylhexanoate 20% 25% 20% Hydroxyethyl cellulose 20% 23% 17.5% 3-Hydroxy-6-phenyl-3,5-hexadien-2-one 5% 10% 0% total 100% 100% 100%
[0037] Squalane, glyceryl triethylhexanoate and hydroxyethyl cellulose can be purchased directly from the market.
[0038] Test groups 1 and 2 have a light herbal-like aroma with tobacco and bean aroma, while test group 3 has no such aroma. In addition, the evaluation team tested the compositions of test group 1, test group 2 and test group 3 in an open environment. 0h , T 72 , T 288h The olfactory evaluation of the test showed that the intensity of the fragrance decreased wit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com