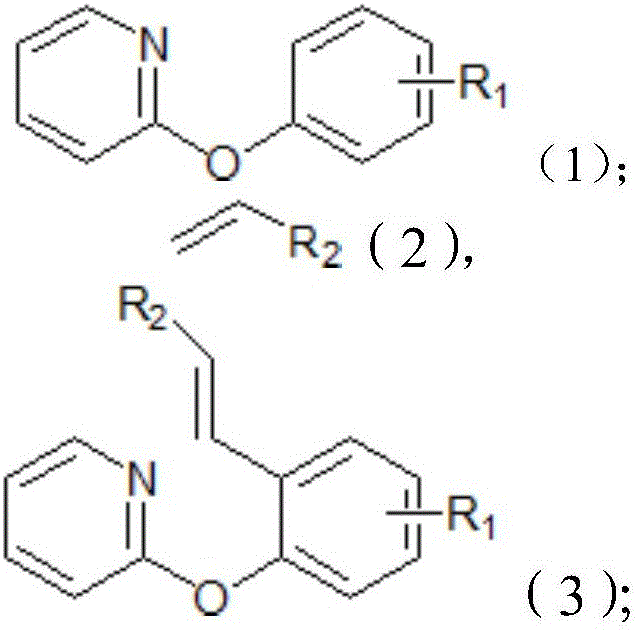
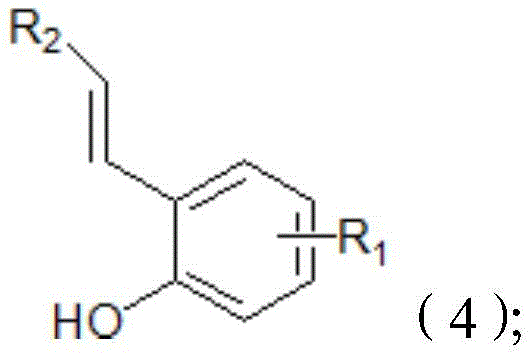
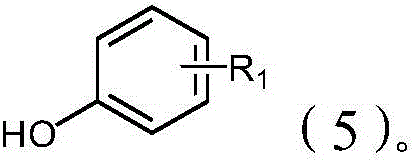
Method for synthesizing o-alkenyl phenol derivate
A technology of o-alkenyl phenol and alkenyl phenol, which is applied in the field of synthesizing o-alkenyl phenol derivatives, can solve problems such as complex reactions and limited product structure diversity, and achieve simple reaction steps, strong reactivity, and high yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037]
[0038] In this example: R 1 is methyl; R 2 For ethoxy.
[0039] Step: In 10mL reaction bottle, add compound I-1 (0.3mmol), ethyl acrylate (0.45mmol), [RhCp*Cl 2 ] 2 (5mol%), AgSbF 6 (20mol%), Cu(OAc) 2(20mol%) and DMF (1mL), the mixture was reacted at 80°C, and TLC (thin-layer chromatography) detected that the reaction was complete. Post-processing purification: cooling to room temperature, concentration under reduced pressure. The crude product was separated and purified by silica gel column chromatography, and the mobile phase was a mixed solvent of V (petroleum ether): V (ethyl acetate) = 5:1 to obtain a pure product, a white solid, with a yield of 92%.
[0040] 1 H NMR (400MHz, CDCl 3 )δ8.20–8.15(m,1H),7.85(d,J=16.1Hz,1H),7.73-7.68(m,1H),7.56(d,J=8.0Hz,1H),7.04(d,J =8.0Hz, 1H), 7.01-7.69(m, 2H), 6.90(s, 1H), 6.43(d, J=16.1Hz, 1H), 4.20(q, J=7.1Hz, 2H), 2.35(s ,3H),1.28(t,J=7.1Hz,3H).
Embodiment 2
[0042]
[0043] In this example: R 1 is methoxy; R 2 For ethoxy.
[0044] Step: In a 10mL reaction flask, add compound I-2 (0.3mmol), ethyl acrylate (0.45mmol), [RhCp*Cl 2 ] 2 (5mol%), AgSbF 6 (20mol%), Cu(OAc) 2 (20mol%) and DMF (1mL), the mixture was reacted at 80°C, and TLC (thin-layer chromatography) detected that the reaction was complete. Post-processing purification: cooling to room temperature, concentrated under pressure. The crude product was separated and purified by silica gel column chromatography, the mobile phase was a mixed solvent of V (petroleum ether): V (ethyl acetate) = 5:1, and the pure product was obtained as a yellow liquid with a yield of 88%.
[0045] 1 H NMR (400MHz, CDCl 3 )δ8.18(dd, J=4.7,1.6Hz,1H),7.82(d,J=16.1Hz,1H),7.72(ddd,J=8.3,7.3,2.0Hz,1H),7.61(d,J =8.8Hz,1H),7.03-6.97(m,2H),6.79(dd,J=8.8,2.6Hz,1H),6.62(d,J=2.5Hz,1H),6.36(d,J=16.1Hz ,1H), 4.19(d,J=7.1Hz,2H),3.79(s,3H),1.28(t,J=7.1Hz,3H).
Embodiment 3
[0047]
[0048] In this example: R 1 is N,N-dimethyl; R 2 Ethoxy.
[0049] Step: In a 10mL reaction flask, add compound I-3 (0.3mmol), ethyl acrylate (0.45mmol), [RhCp*Cl 2 ] 2 (5mol%), AgSbF 6 (20mol%), Cu(OAc) 2 (20mol%) and DMF (1mL), the mixture was reacted at 80°C, and TLC (thin-layer chromatography) detected that the reaction was complete. Post-processing purification: cooling to room temperature, concentrated under pressure. The crude product was separated and purified by silica gel column chromatography, and the mobile phase was a mixed solvent of V (petroleum ether): V (ethyl acetate) = 5:1 to obtain a pure product, a yellow solid, with a yield of 85%.
[0050] Compound II-3 was tested:
[0051] 1 H NMR (400MHz, CDCl 3 )δ8.19(d,J=5.0Hz,1H),7.76(d,J=16.0Hz,1H),7.72–7.65(m,1H),7.55(d,J=8.9Hz,1H),7.01– 6.94(m,1H),6.92(d,J=8.3Hz,1H),6.56(dd,J=8.9,2.4Hz,1H),6.33(d,J=2.6Hz,1H),6.25(d,J =16.0Hz, 1H), 4.17(q, J=7.1Hz, 2H), 2.97(s, 6H), 1.26(t, J=7.1Hz, 3H).
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com