Electrochemical measuring method of ferric iron content
A determination method and electrochemical technology, applied in the direction of electrochemical variables of materials, etc., can solve the problems of quantitative analysis trouble, ecological environment hazards, affecting analysis performance, etc., and achieve the effect of reducing historical residual impurities, avoiding saturation reactions, and improving sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] In this example, HOKUTO DENKO HAB-151 differential chronopotential analyzer and electrochemical three-electrode test system are used. A gold electrode is used as a working electrode, platinum wire is used as a counter electrode, and Ag / AgCl is used as a reference electrode. A constant current anode is used to dissolve the differential chronopotential The content of ferric iron in the samples was tested by CCSCP method. Reduction enrichment and anodic dissolution conditions are respectively: enrichment current is -4.0μA, enrichment time is 180s, resting time is 30s; during the test, anodic stripping scanning current is +5.0μA, scanning rate is 20mV s -1 , the frequency is 20Hz.
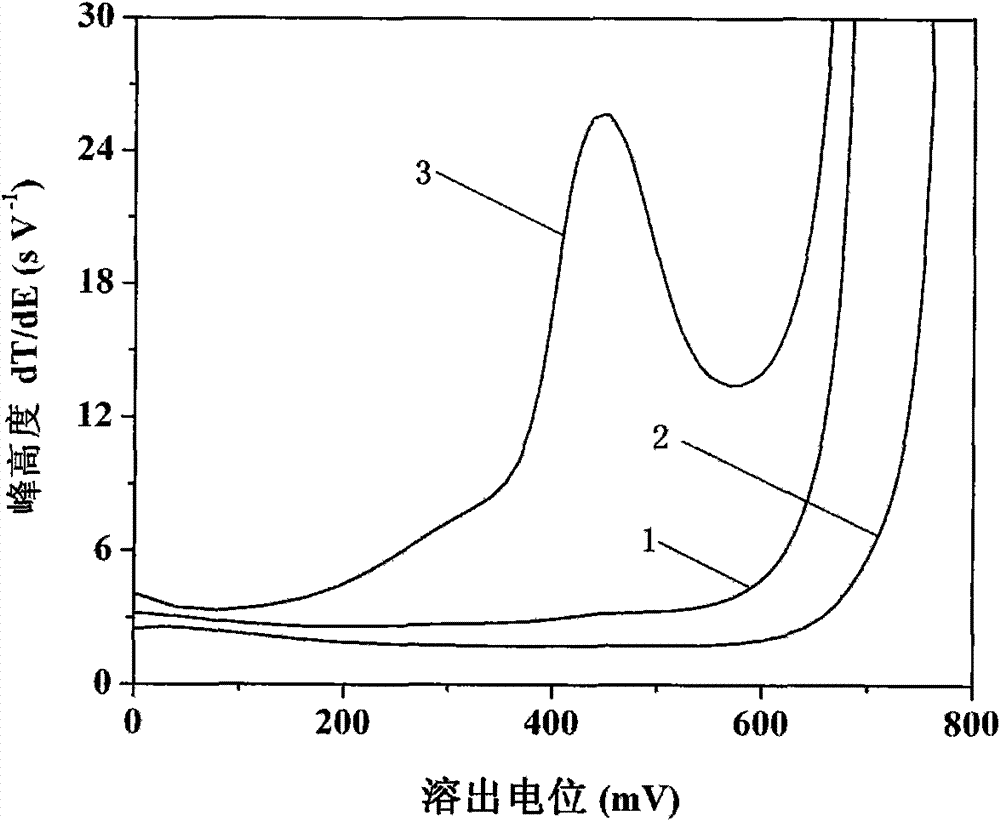
[0024] See attached figure 1 , curve 1 in the figure is for 5.0mmol L by the above method -1 Sodium acetate+80mmol L -1 Hydroxylamine hydrochloride+15.0mmol L -1 The o-phenanthroline solution contains 0.2mol L -1 The result that test obtains after the propylene carbonate extraction of TBAP;...
Embodiment 2
[0028] In this example, HOKUTO DENKO HAB-151 differential chronopotential analyzer and electrochemical three-electrode test system are used. A gold electrode is used as a working electrode, platinum wire is used as a counter electrode, and Ag / AgCl is used as a reference electrode. A constant current anode is used to dissolve the differential chronopotential The method (CCSCP) was used to determine the content of ferric iron in the spiked seawater. Reduction enrichment and anodic dissolution conditions are respectively: enrichment current is -5.0μA, enrichment time is 180s, resting time is 30s; during the test, anodic stripping scanning current is +5.0μA, scanning rate is 20mV s -1 , the frequency is 20Hz.
[0029] Fresh actual seawater samples were first filtered through 0.45 μm microporous filter paper.
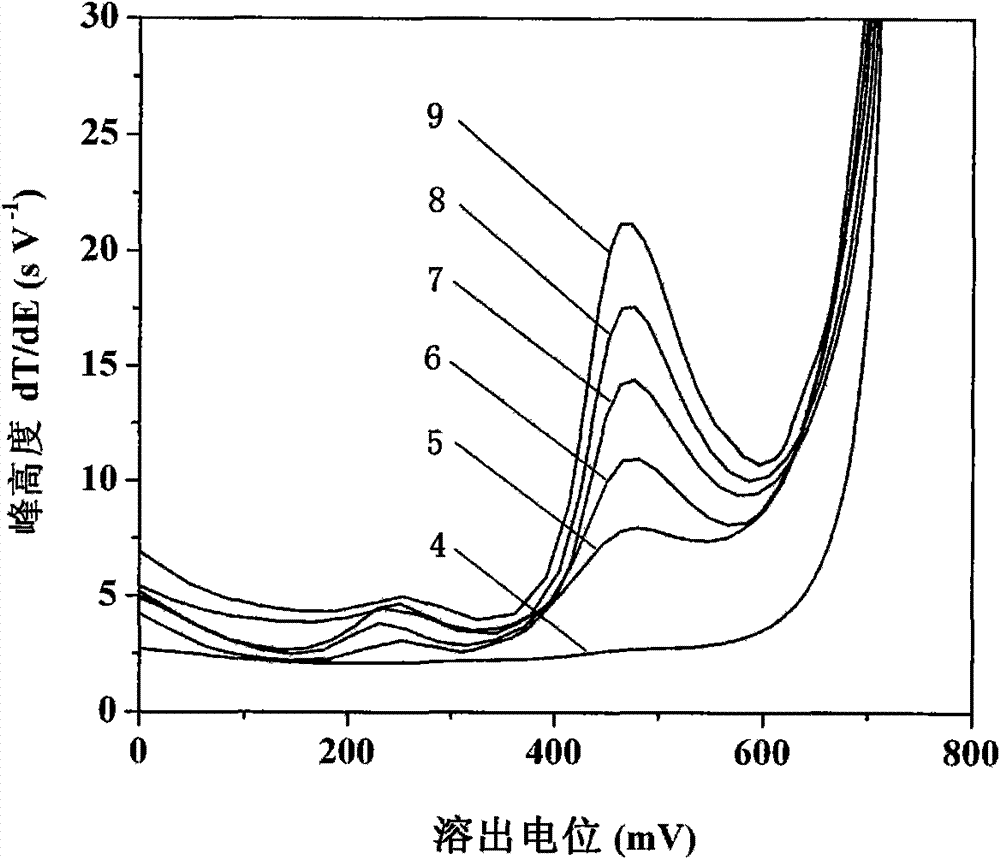
[0030] See attached figure 2 , first with 5.0mmol L -1 Sodium acetate, 1.0mol L -1 NaCl, 80mmolL -1 Hydroxylamine hydrochloride, 7.5mmol L -1 The 10mL bottom solution...
Embodiment 3
[0033] According to the test method of Example 2, the iron content of the spiked river water was measured.
[0034] Fresh actual river water samples were first filtered through 0.45 μm microporous filter paper.
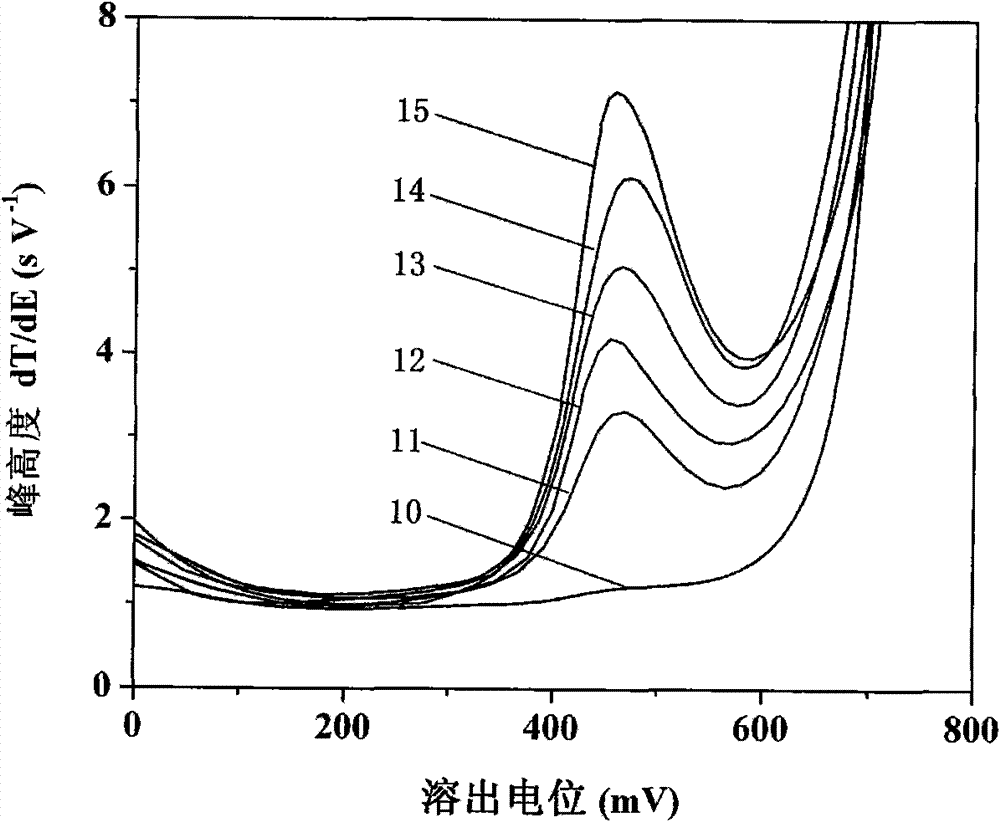
[0035] See attached image 3 , first with 5.0mmol L -1 Sodium acetate, 1.0mol L -1 NaCl, 80mmolL -1 Hydroxylamine hydrochloride, 7.5mmol L -1 The 10mL bottom solution of o-phenanthroline contains 0.1mol L -1 Test after the propylene carbonate extraction of TBAP, the result is shown by curve 10; In curve 10 solution, add 10mL sampling river water after treatment, adjust and keep the equilibrium concentration of each component to be: 5.0mmol L -1 Sodium acetate, 1.0mol L -1 NaCl, 80mmolL -1 Hydroxylamine hydrochloride, 7.5mmolL -1 O-phenanthroline, then containing 0.1mol L -1 After the propylene carbonate extraction of TBAP was tested, the result was shown by curve 11; -1 The concentration gradient of Fe(III) was added standardly, and test curves 12-15 were ob...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com