Catalyst system for use in preparation of acetic acid and acetic anhydride or synchronous preparation of acetic acid and acetic anhydride and preparation method thereof
A technology of acetic anhydride and catalyst, applied in the field of catalyst system and preparation thereof, can solve the problems of easy precipitation, rising production cost, poor product purity, etc., and achieve the effects of reduced corrosion, reduced manufacturing cost and improved stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A
[0044] Add rhodium precursor rhodium iodide and iridium iodide in the reaction still prepared by catalyst, make the mass ratio of rhodium and iridium be (2~10):(5~20), add the N with iridium mole fraction 1:1 -Methylalanine and water; add methyl iodide and acetic acid as a solvent; then pass through CO, and stir for 2 hours at a reaction temperature of 150° C. and a pressure of 3.0 MPa. After cooling, it is detected with an infrared spectrometer that rhodium in the catalyst solution is [Rh(CO) 2 I 2 ] - Exists in the form of iridium [Ir(CO) 2 I 2 ] - form exists.
Embodiment B
[0046] Add the rhodium precursor rhodium iodide in the reaction kettle prepared by the catalyst, then add methyl iodide and solvent acetic acid, then pass into CO, and react for 2 hours at a reaction temperature of 150° C. and a pressure of 3.0 MPa to prepare a rhodium coordination compound. This coordination compound is in the form of a solution.
[0047] This example is not an example of the invention because there is no amino acid or amino acid derivative stabilizer in the catalyst system.
Embodiment 1
[0049] The dosage of each raw material in the catalyst system is: 8 parts by weight of rhodium iodide; 10 parts by weight of iridium iodide; 1200 parts by weight of methyl iodide; the molar ratio of N-methylalanine to iridium is 1.0; and 2.5 parts by weight of lithium acetate.
[0050] Prepared by the method described in Example A.
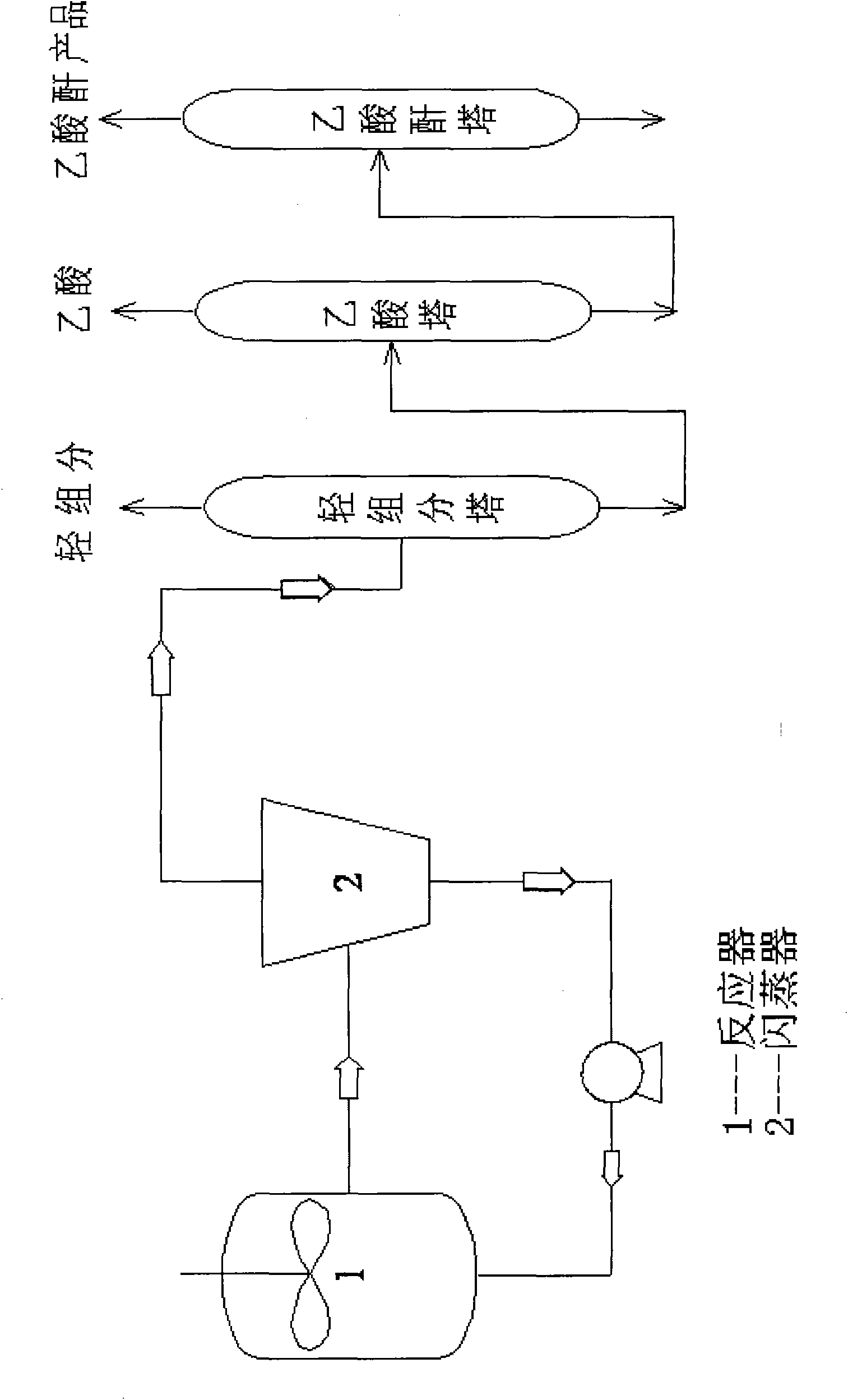
[0051] in the process as attached figure 1 In the continuous device of the present invention, the above-mentioned product is added in the reactor together with methyl iodide, acetic acid, acetic anhydride, lithium acetate, and the addition amount of methyl iodide, acetic acid, acetic anhydride is determined according to reaction needs, and the purpose of acetic anhydride consumes the water added in the catalyst preparation . The reaction temperature is 190°C, the pressure is 4.0MPa, the mass concentration of rhodium in the control reaction solution is 800ppm, the mass concentration of iridium is 1000ppm, the mass concentration of lithium is 250pp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com