Method for directly synthesizing nickel phthalocyanine crystal by using solvent thermal method
A nickel phthalocyanine, solvothermal technology, applied in crystal growth, chemical instruments and methods, solutions of liquid solvents at room temperature, etc., can solve the problems of long growth cycle, small crystal size, low success rate, etc., and achieve short reaction time. , low cost and simple equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
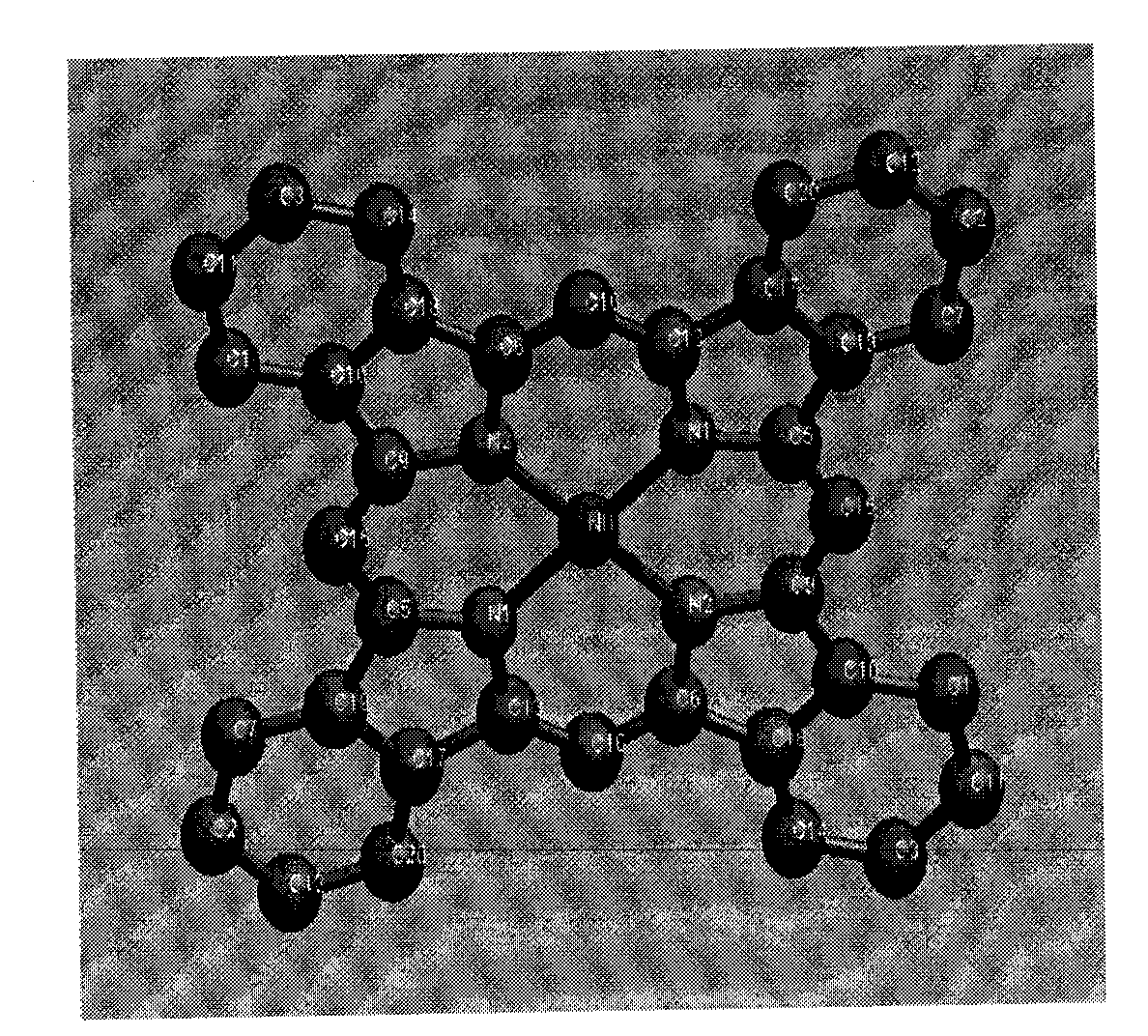
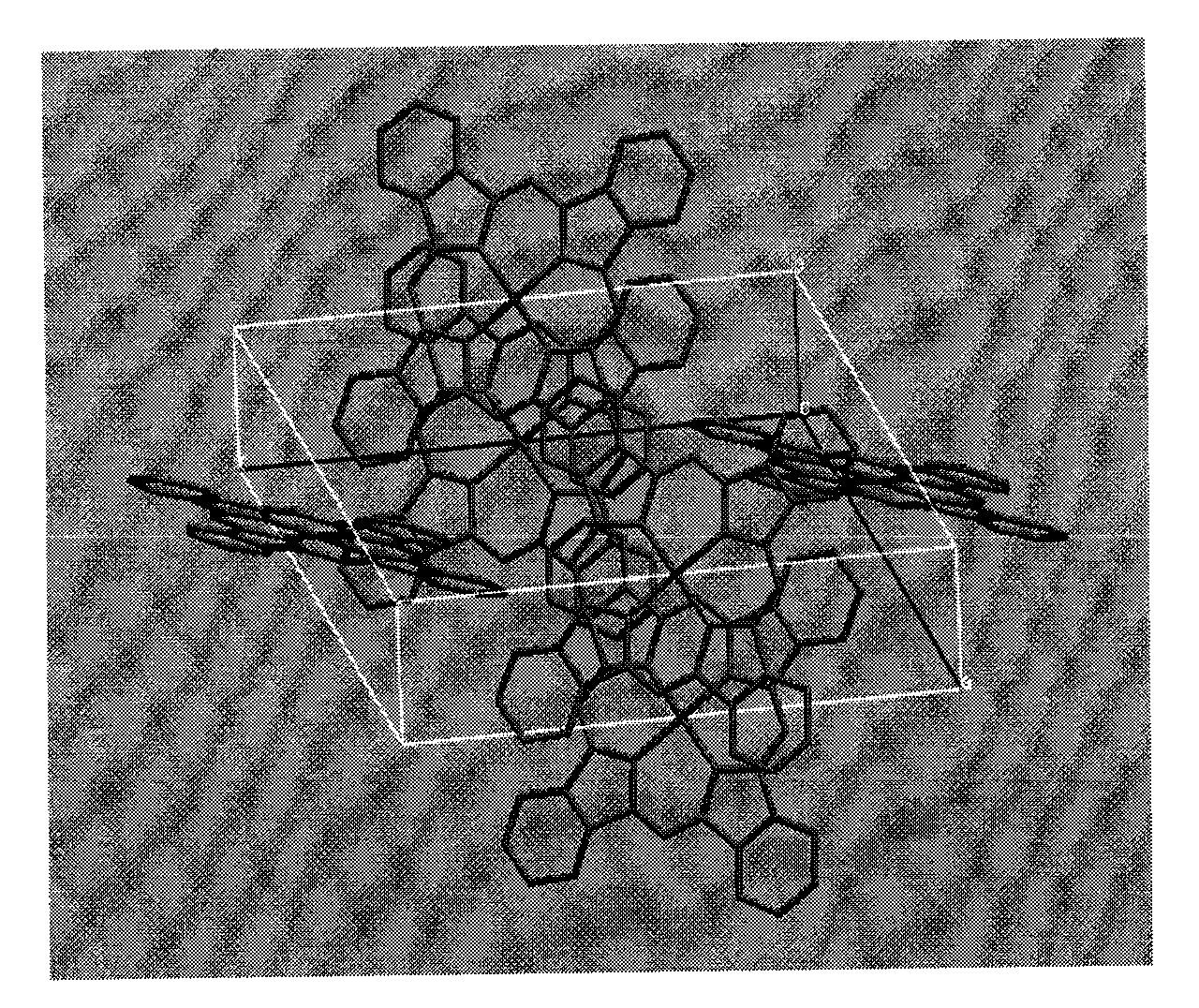
[0017] A method for directly synthesizing nickel phthalocyanine crystals with a solvothermal method, comprising the following steps: taking quinoline as a solvent, nickel acetate dihydrate and indole as starting materials, and nickel acetate dihydrate and indole in moles Add the steel bomb at a ratio of 1:2, then add the quinoline solvent, tighten the steel bomb and put it in an oven, heat it to 200°C, react for 7 hours, and cool it to room temperature; finally filter and rinse with methanol, that is Purple needle-shaped nickel phthalocyanine crystals can be directly obtained with a yield of 70%.
Embodiment 2
[0019] A method for directly synthesizing nickel phthalocyanine crystals with a solvothermal method, comprising the following steps: taking quinoline as a solvent, nickel acetate dihydrate and indole as starting materials, and nickel acetate dihydrate and indole in moles Add the ratio of 1:5 to the reaction kettle, then add the quinoline solvent, react at a temperature of 300°C for 10 hours, and cool to room temperature; finally filter and rinse with methanol, and the purple needle-shaped phthalocyanine can be directly obtained Nickel crystals, 79% yield.
Embodiment 3
[0021] A method for directly synthesizing nickel phthalocyanine crystals with a solvothermal method, comprising the following steps: taking quinoline as a solvent, nickel acetate dihydrate and indole as starting materials, and nickel acetate dihydrate and indole in moles The ratio of 1:4 was added to the reaction kettle, and then quinoline solvent was added, and the temperature was 270 °C for 9 hours, and then cooled to room temperature; finally filtered, rinsed with deionized water, and the purple needle-shaped Nickel phthalocyanine crystals with a yield of 75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com