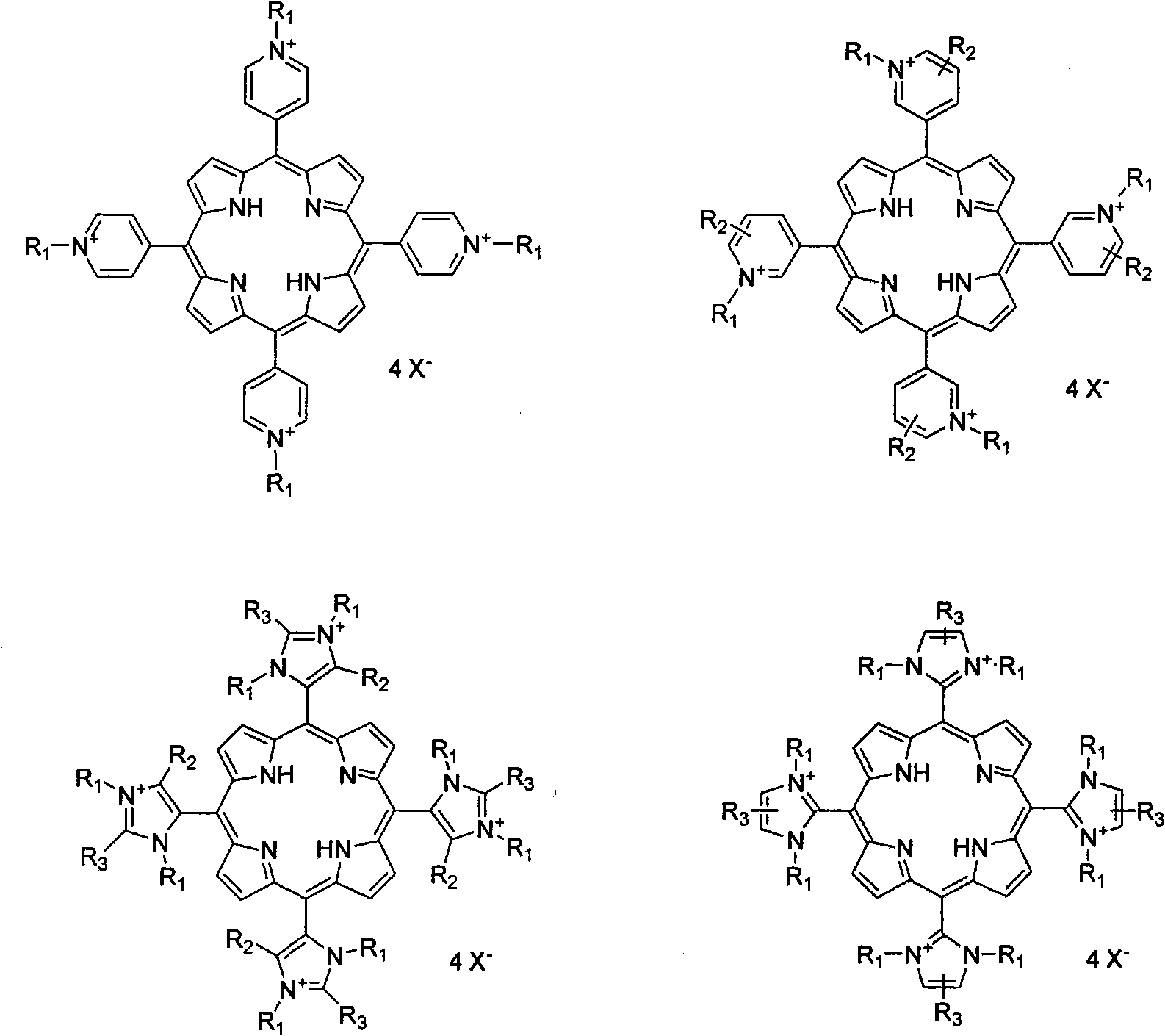
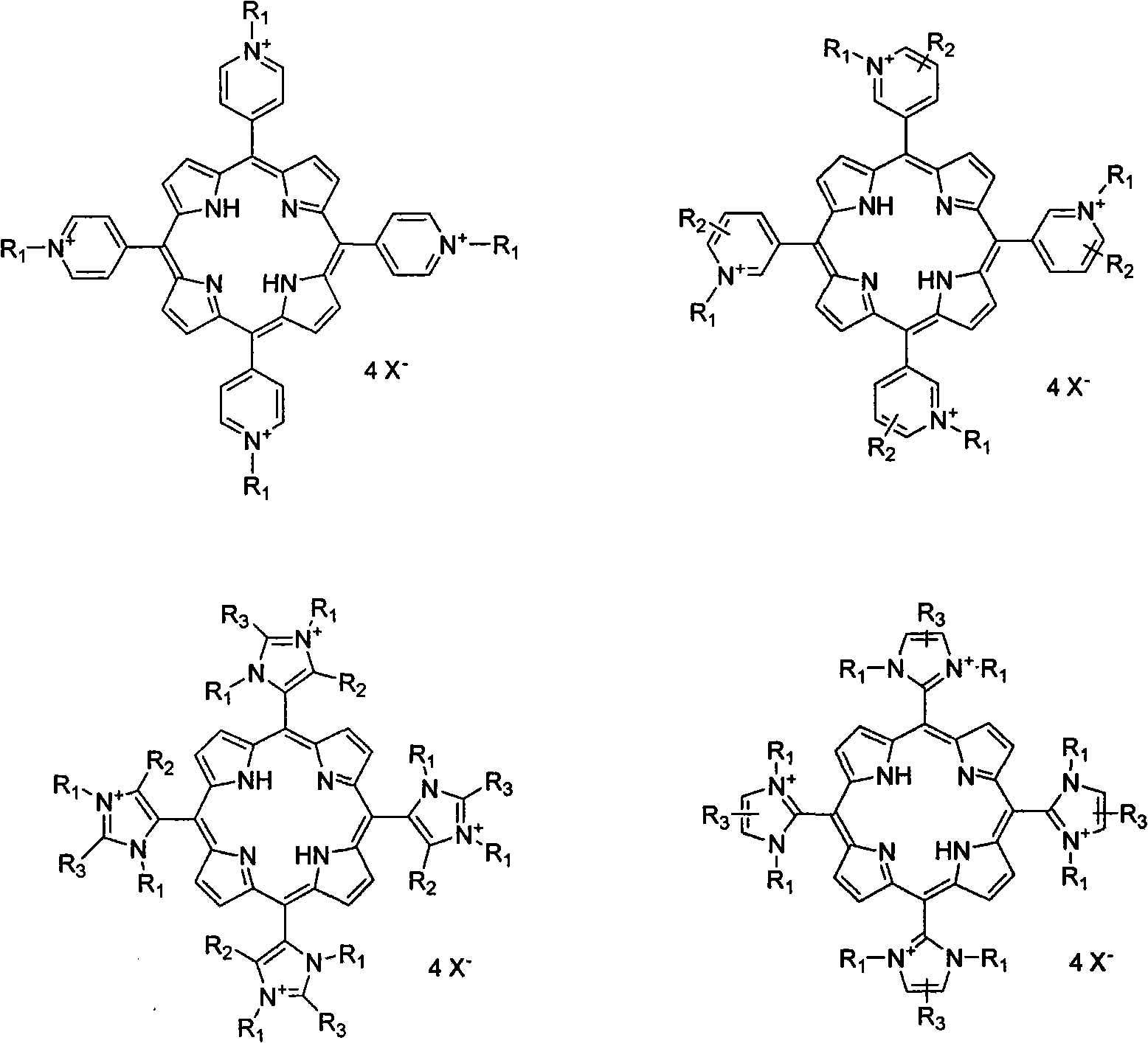
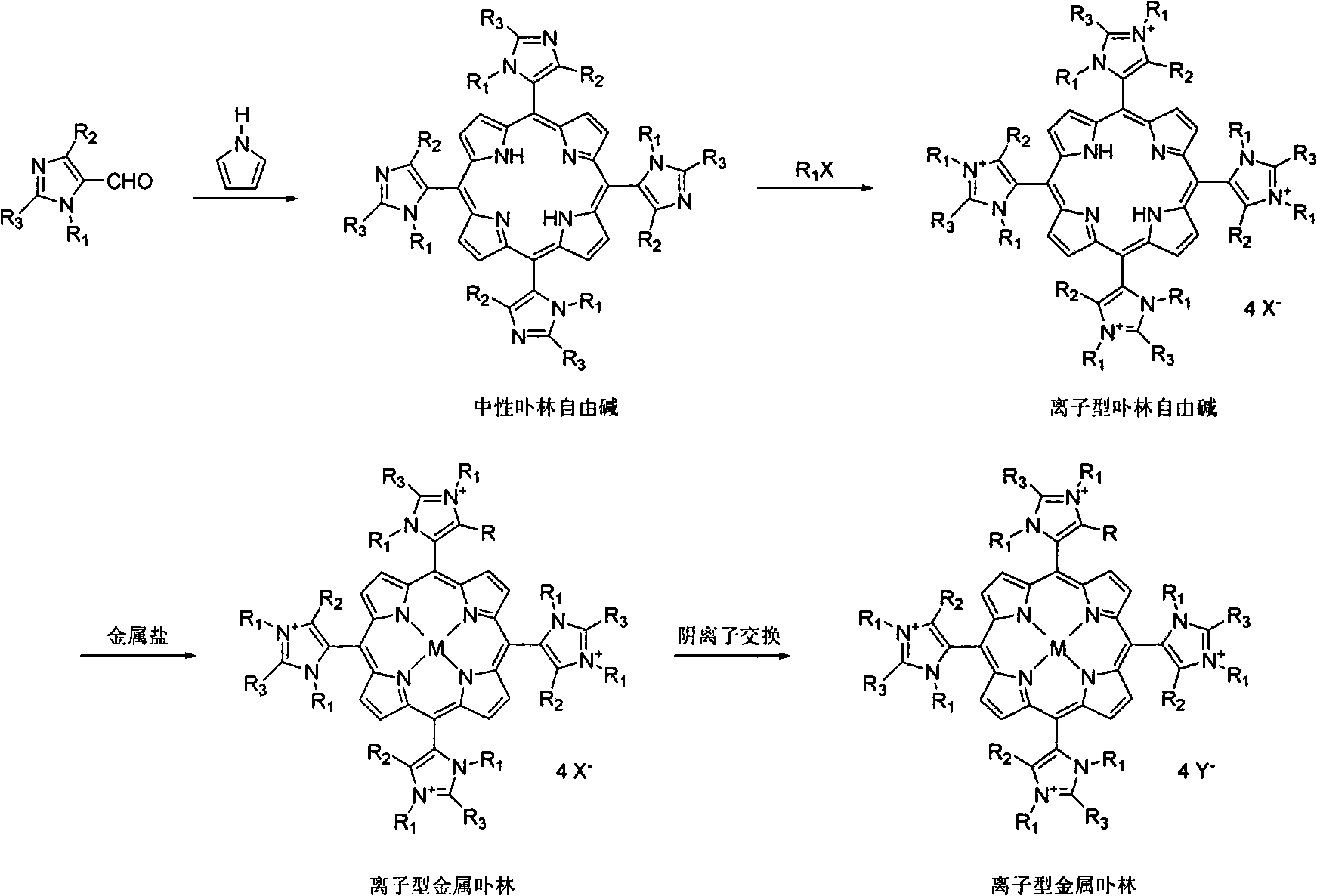
Method for synthesizing ionic type metal porphyrin
A synthesis method and technology of metalloporphyrins, applied in the direction of organic chemistry, etc., can solve the problems of difficult porphyrin separation and purification, low separation yield, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Add 500mL propionic acid, 12.8g 4-pyridine formaldehyde, and 8.0g pyrrole in a 1000mL three-necked flask, and reflux for 2.5h. After the reaction is completed, remove most of the propionic acid by rotary evaporation, then add 600mL ethanol, cool and recrystallize to obtain four Pyridyl porphyrin, the yield is 26.0%. The product was added with 50 mL of N, N-dimethylformamide, and excess iodomethane, refluxed for 2 hours, and the reaction mixture was filtered to obtain tetrakis-(N-methyl-4-pyridyl)porphyrin iodide salt with a yield of 90.2%. The obtained solid was dissolved in 50 mL of deionized water, an excess of manganese acetate was added, refluxed for 2 hours, and then ammonium hexafluorophosphate was added, and the obtained solid was precipitated as tetrakis-(N-methyl-4-pyridyl)manganese porphyrin hexafluorophosphate , yield 99.0%.
Embodiment 2
[0027] Dissolve the tetrakis-(N-methyl-4-pyridyl)porphyrin iodide salt synthesized according to the steps of Example 1 in 50 mL of deionized water, add excess palladium chloride, reflux for 2 hours, cool and filter to remove the excess insoluble metal salt , ammonium hexafluorophosphate was added to the filtrate, and the precipitate was tetrakis-(N-methyl-4-pyridyl)palladium porphyrin hexafluorophosphate, with a yield of 92.0%.
Embodiment 3
[0029] The four-(N-methyl-4-pyridyl) porphyrin iodide salt synthesized according to the steps of Example 1 was dissolved in 50 mL of deionized water, an excess of platinum chloride was added, refluxed for 2 hours, cooled and filtered to remove the excess metal salt, Ammonium hexafluorophosphate was added to the filtrate, and the precipitate was tetrakis-(N-methylpyr-4-idinyl)palladium porphyrin hexafluorophosphate, with a yield of 93.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com