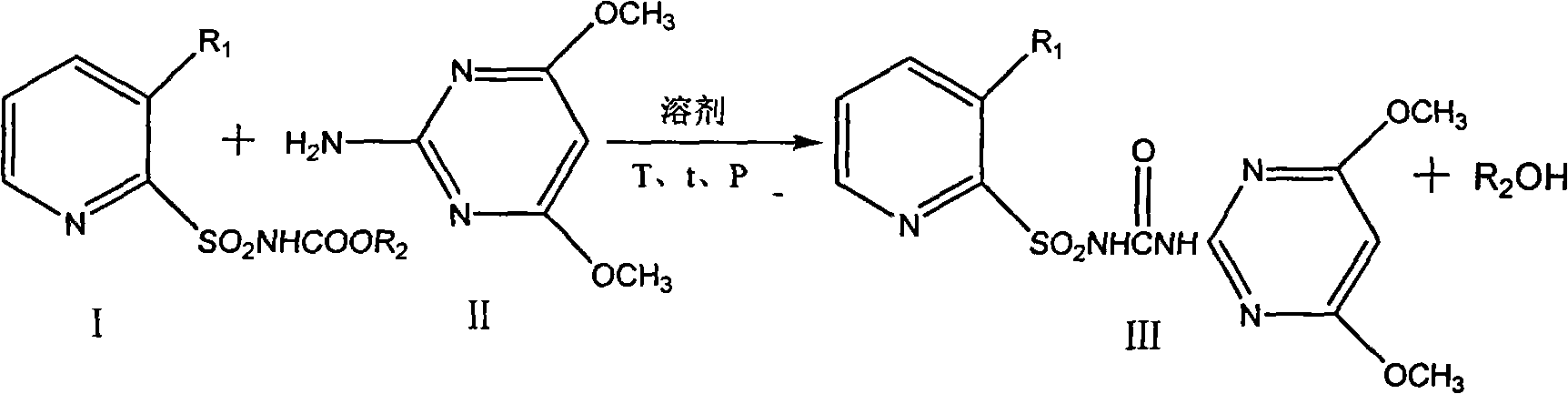
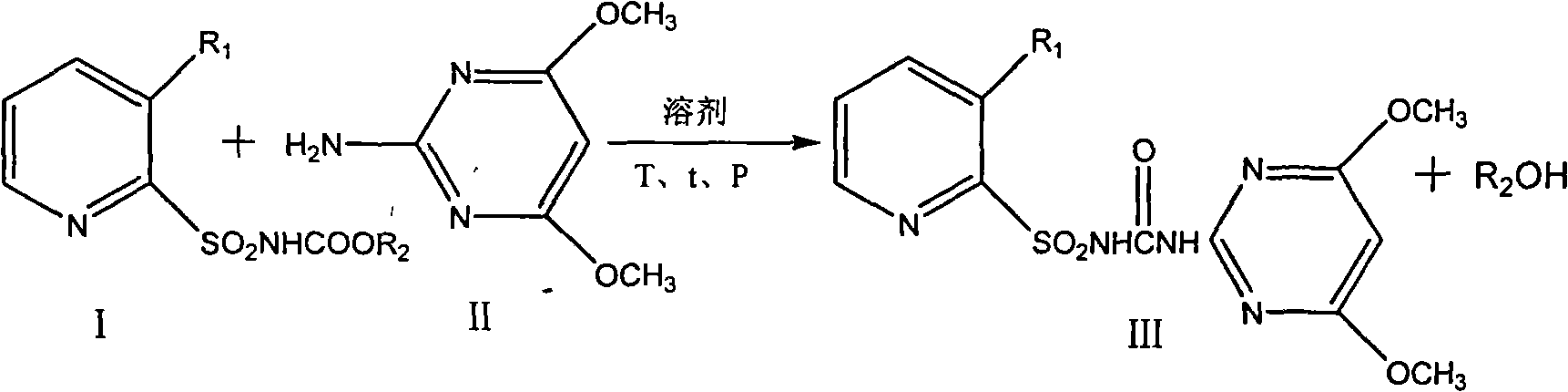
Novel synthesis method of sulfonylurea weedicide
A herbicide and sulfonylurea technology, applied in the field of fine organic chemistry, can solve problems such as low yield, high reaction temperature, and many side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Embodiment 1, the preparation of nicosulfuron
[0014] 23.3kg 150mol 2-amino-4,6-dimethoxypyrimidine, 30.1kg 100mol 2-sulfonamide ethyl formate-N,N-dimethylnicotinamide were put into the reaction kettle, and then 200L toluene was added, at 90℃ Under reduced pressure, the reaction was rectified under reduced pressure, ethanol was continuously removed, and the reaction of ethyl 2-sulfonamide formate-N, N-dimethylnicotinamide in the central control was complete. The temperature was lowered to 80°C, filtered, and dried. The yield of nicosulfuron was 99.5 % (calculated as pyridine sulfonamidate), the purity is 98.4%.
Embodiment 2
[0015] Embodiment 2, the preparation of nicosulfuron
[0016] The operation is the same as in Example 1, replacing 30.1kg 100mol ethyl 2-sulfonamide formate-N,N-dimethylnicotinamide with 28.7kg 100mol methyl 2-sulfonamide formate-N,N-dimethylnicotinamide, Results The yield of nicosulfuron was 96.2%, and the purity was 95.9%.
Embodiment 3
[0017] Embodiment 3, the preparation of pyrasulfuron-methyl
[0018] 23.3kg 150mol 2-amino-4,6-dimethoxypyrimidine, 29.8kg 100mol 2-sulfonamidoformic acid ethyl ester-3-trifluoromethylpyridine were put into the reaction kettle, then 200L toluene was added, and at 90°C, Rectification under reduced pressure, ethanol was continuously removed, and the reaction of ethyl 2-sulfonamidoformate-3-trifluoromethylpyridine in the central control was complete. The temperature was lowered to 80°C, filtered, and dried. The yield of nicosulfuron was 95.3%, and the purity was 96.7% %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com