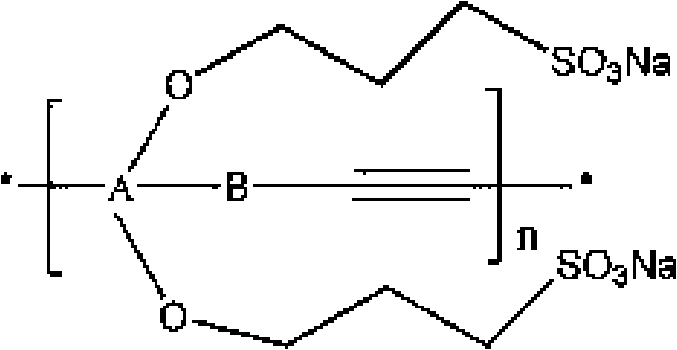
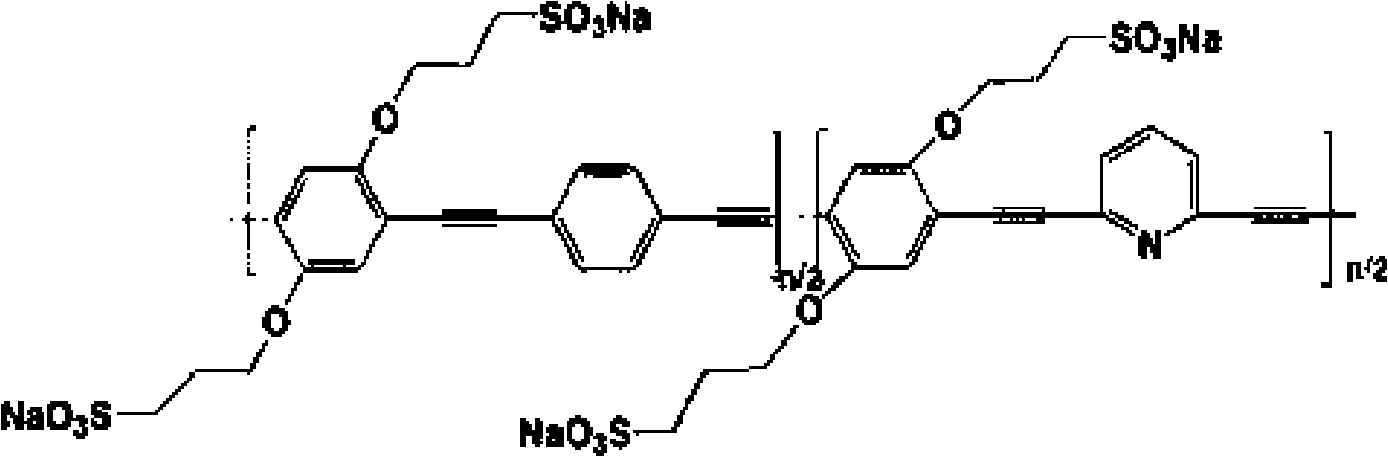
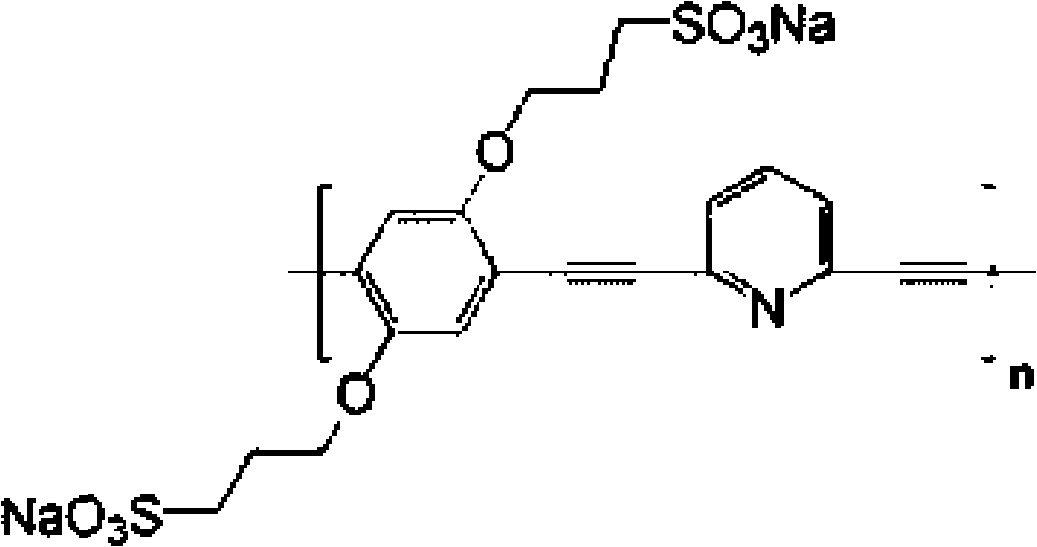
Water soluble fluorescent conjugated polymer and synthesis method thereof
A technology of conjugated polymers and synthesis methods, applied in chemical instruments and methods, luminescent materials, etc., can solve problems such as poor selectivity and poor specificity, achieve excellent optical properties, improve rigidity, and increase flexibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Preparation of raw materials:
[0025] A: Solvent preparation: (5ml of water, 7.5ml of dimethylformamide, 2.5ml of diisopropylamine) mix evenly and deoxygenate the solution with nitrogen for ten minutes;
[0026] B: In a 100ml three-necked flask, under nitrogen protection, add 0.1342g (0.21mmol) of 1,4-diiodo-2,5-dipropoxysodium sulfonate)benzene, 1,4-diynylbenzene 0.0120g (0.12mmol) g, 0.0134g (0.1mmol) of 2,6-diynylpyridine, 12mg (10.0μmol) of tetrakistriphenylphosphopalladium, CuI, 2mg (10.0μmol) under the protection of nitrogen, and then added with a syringe the above solvents;
[0027] (1) Polymerization: react at 65°C for 24 hours, the solution is dark brown, and has bright blue fluorescence under the irradiation of ultraviolet light;
[0028] (2) Centrifuge to separate impurities: after cooling to room temperature, centrifuge to remove insoluble matter;
[0029] (3) Precipitation: the solution is slowly added to 125ml of acetone / ether (75 / 50) mixed solution st...
Embodiment 2
[0032] Solvent preparation: 5ml of water, 7.5ml of DMF, 2.5ml of diisopropylamine, deoxygenated by nitrogen for ten minutes;
[0033] In a 100ml three-necked flask, under nitrogen protection, add 1,4-diiodo-2,5-dipropoxysodium sulfonate)benzene 0.0663g (0.102mmol), 2,6-diynylpyridine 0.0127g (0.10mmol), under the protection of nitrogen, tetrakistriphenylphosphopalladium 12mg (10.0μmol), CuI, 2mg (10.0μmol), the solvent was added with a syringe, and the solution was dark brown at 65°C for 24 hours. Under irradiation, there is bright blue fluorescence. After cooling to room temperature, filter to remove insoluble matter, spin the filtrate to concentrate, slowly add to 125ml high-speed stirred acetone / ether (75 / 50) mixed solution, a large amount of dark brown precipitate appears, stand still, remove the supernatant, The lower layer was centrifuged, and the solid was redissolved with a small amount of methanol, and then settled twice in the mixed solution of acetone / ether to obta...
Embodiment 3
[0038] Water 7.5ml, DMF 11.25ml, diisopropylamine 3.75ml, nitrogen deoxygenation for 10 minutes, in a 100ml three-necked flask, under the protection of nitrogen, add 1,4-diiodo-2,5-dipropoxysulfonate Sodium acid) benzene 0.2023g (0.31mmol), 1,4-diynylbenzene 0.0165 (0.16mmol) g, 2,6-diynylpyridine 0.0203g (0.16mmol), tetrakistriphenylphosphopalladium 17mg (15.0 μmol), CuI, 3 mg (15.0 μmol) under the protection of nitrogen, add the solvent with a syringe, and react at 65°C for 24 hours, the solution is dark brown, and has bright blue fluorescence under the irradiation of ultraviolet light. After cooling to room temperature, centrifuge to remove insoluble matter, slowly add the solution to 190ml of acetone / ether (115 / 75) mixture stirred at high speed, a large amount of dark brown precipitate appears, stand still, remove the supernatant, and centrifuge the lower layer , after the solid was redissolved with a small amount of methanol, it was settled twice in the mixed solution of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com