New febuxostat crystal form and preparing method thereof
A febuxostat and crystal form technology, applied in bone diseases, organic chemistry, drug combination, etc., can solve problems such as the problem of not describing the crystal form
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The preparation method of febuxostat crystal form X:
[0026] Add 1 g of febuxostat to 6 ml of methyl ethyl ketone, heat to reflux to dissolve, then cool to room temperature while stirring, and filter after stirring for 2 hours. Dry at 60°C. A 0.7 g sample of crystal form X was obtained, with a melting point of 201-204°C.
Embodiment 2
[0028] The preparation method of febuxostat crystal form X:
[0029] Add 5 g of febuxostat into 20 ml of methyl ethyl ketone, heat to reflux to dissolve, then cool to room temperature while stirring, and filter after stirring for 2 hours. Dry at 60°C. A 4.1 g sample of crystal form X was obtained, with a melting point of 201-204°C.
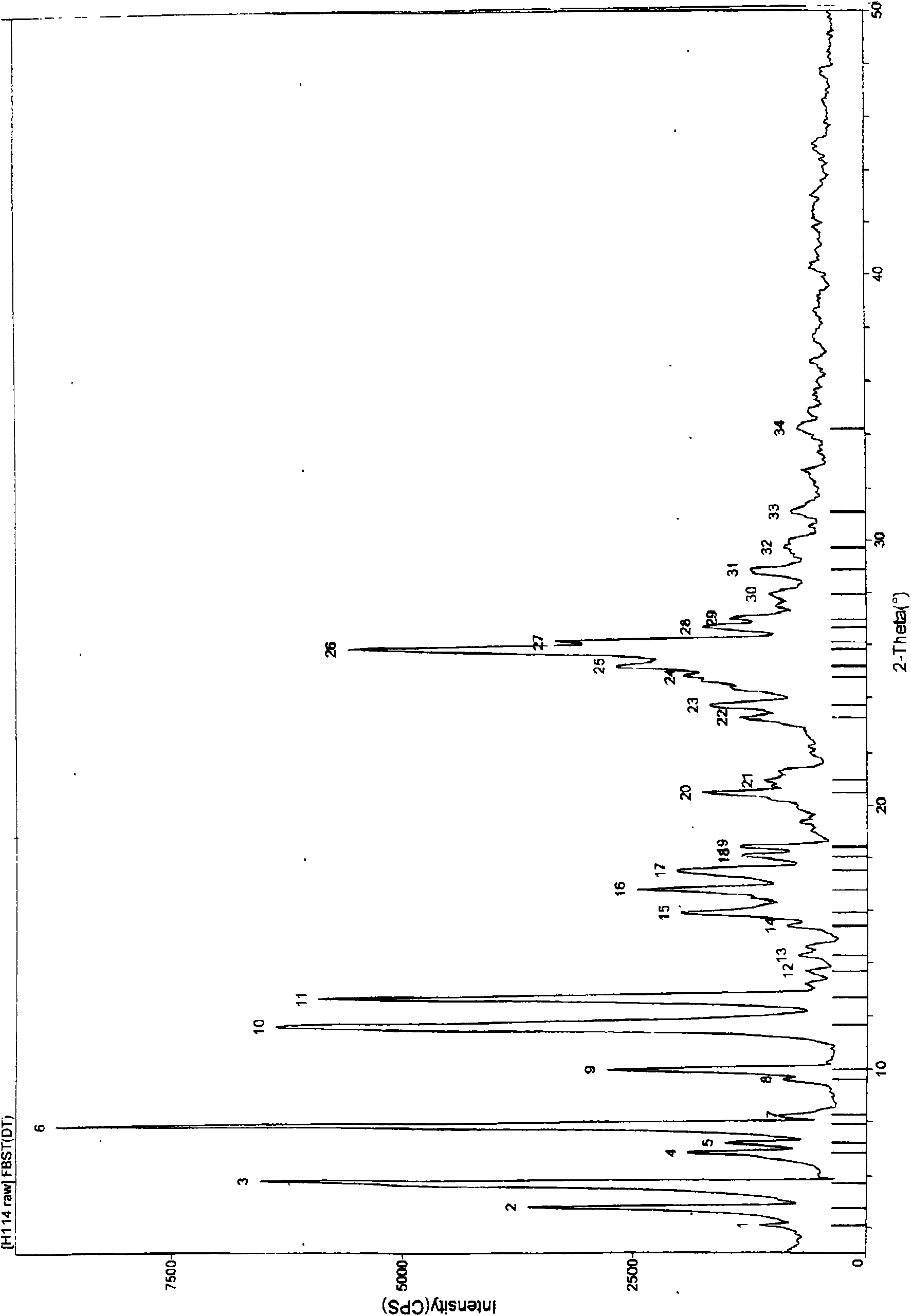
[0030] Form X powder X-ray diffraction 20 is: 4.08, 4.77, 5.73, 6.80, 7.17, 7.91, 8.27, 9.62, 9.99, 11.66, 12.70, 13.70, 14.30, 15.42, 15.90, 16.80, 17.51, 18.06, 18.42, 20.48 , 20.95, 23.27, 23.72, 24.78, 25.18, 25.82, 26.10, 26.64, 26.96, 27.90, 28.85, 29.70, 31.08, 34.18
Embodiment 3
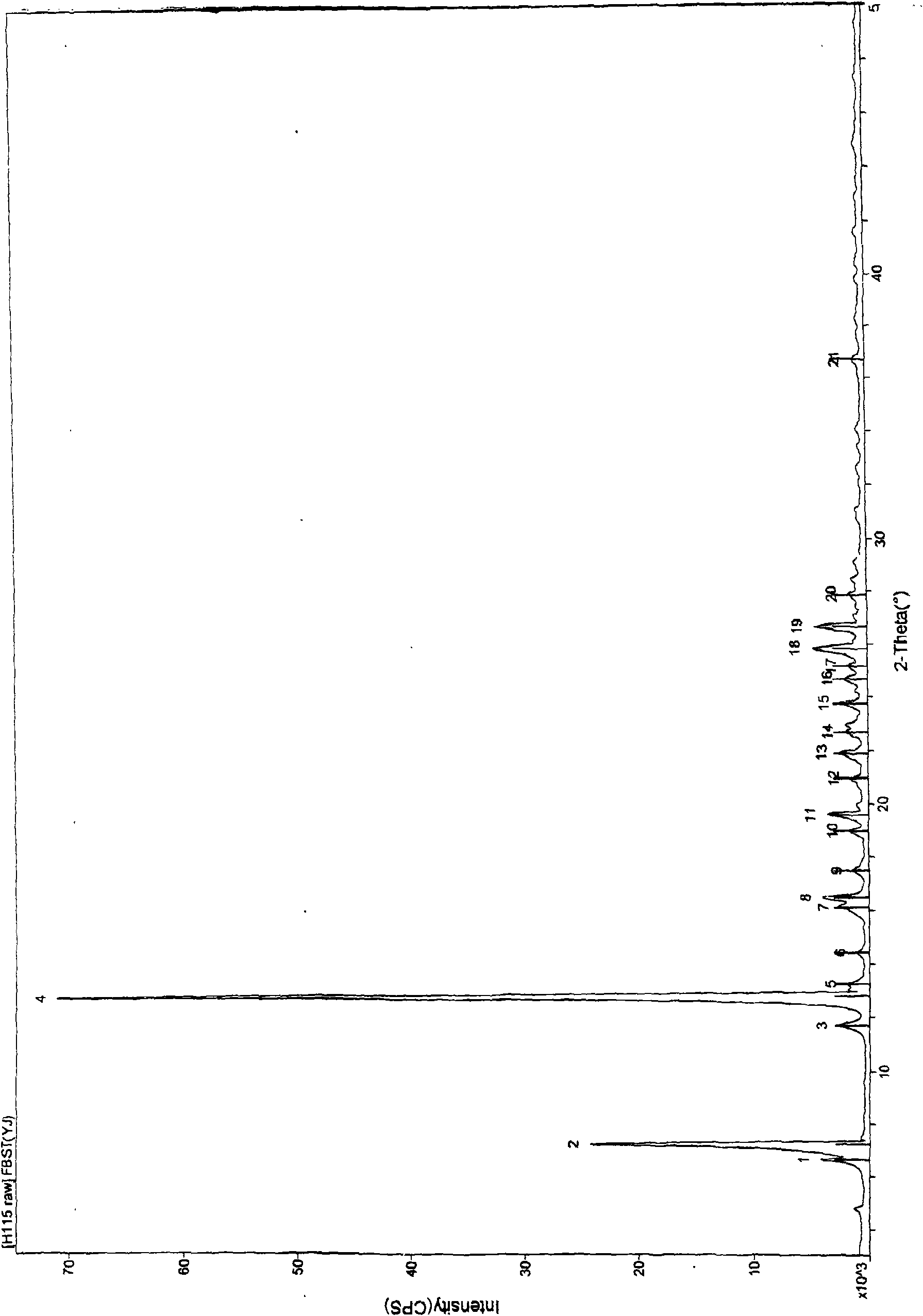
[0032] The preparation method of febuxostat crystal form Y:
[0033] Add 2 g of febuxostat to 5 ml of acetone and reflux to dissolve, then add 10 ml of acetonitrile dropwise under stirring and reflux, cool to room temperature after the addition, stir for 1 hour, and filter. Dry at 60°C. Obtained febuxostat crystal form Y sample 1.4 g, melting point 202-204 ° C
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com