Spiraling whitefly specificity SCAR primer, detecting method and reagent case thereof
A detection kit and the technology of screw whitefly, applied in the field of molecular biology, can solve the problems of no detection method for screw whitefly, and achieve the effects of saving detection time, simple operation process and high detection accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
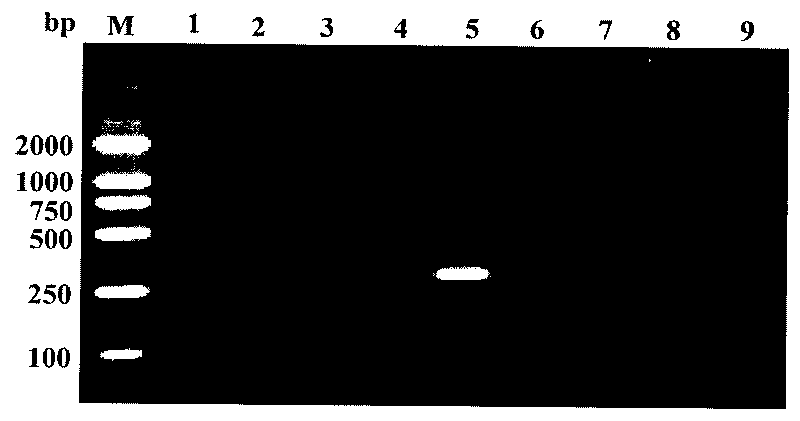
[0034] Example 1: Amplification effect of primer ADZE / ADZF on spiral whitefly
[0035] 1) Preparation of whitefly template DNA
[0036]Place the single-headed whitefly on a parafilm dripped with 20 μL of extraction buffer (50 mmol / L Tris-HCl, 1 mmol / L EDTA, 1% SDS, 20 mmol / L NaCl, pH 8.0), and use the bottom of a 0.2 mL PCR tube as The homogenizer is fully ground, and the homogenate is transferred into a 1.5mL centrifuge tube with a micropipette; then the homogenizer is washed with 100μL buffer solution for 4 times, transferred to the same centrifuge tube, mixed evenly, and 5μL proteinase K (20mg / mL) is added. After mixing thoroughly, place in a water bath at 60°C for 1 hour (mix once in the middle); then bathe in boiling water for 5 minutes, add 220 μL of chloroform / isoamyl alcohol (V:V=24:1) extract, and mix gently for dozens of times. Place on ice for 30 minutes; centrifuge at 4°C and 10,000 r / min for 10 minutes, take the supernatant, add 440 μL pre-cooled absolute ethanol...
Embodiment 2
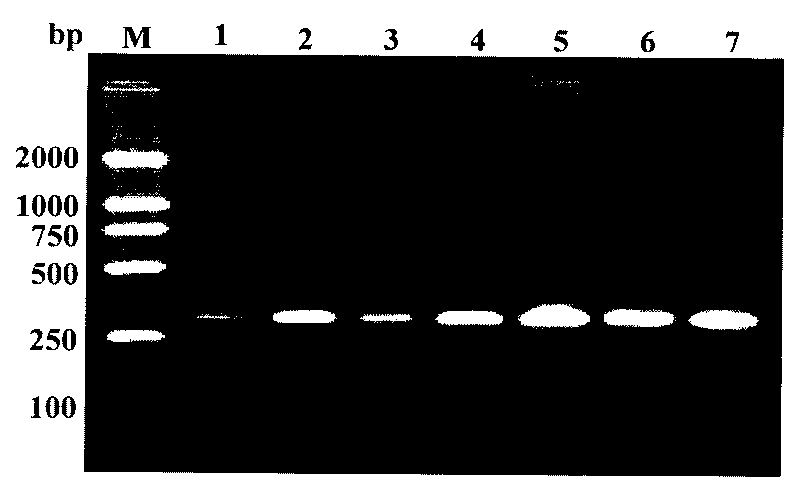
[0054] Example 2: Amplification effect of primers ADZE / ADZF on different stages and sexes of the spiral whitefly
[0055] 1) Preparation of spiral whitefly template
[0056] Single-headed / single-spirited whiteflies of different stages and sexes were placed on a parafilm dripped with 20 μL of extraction buffer (50 mmol / L Tris-HCl, 1 mmol / L EDTA, 1% SDS, 20 mmol / L NaCl, pH 8.0) , using the bottom of a 0.2mL PCR tube as a homogenizer to fully grind, transfer the homogenate into a 1.5mL centrifuge tube with a micropipette; then wash the homogenizer with 100μL buffer solution for 4 times, transfer to the same centrifuge tube, mix well, add 5 μL of proteinase K (20 mg / mL), mix thoroughly, and place in a water bath at 60°C for 1 hour (mix once in the middle); then in a boiling water bath for 5 minutes, add 220 μL of chloroform / isoamyl alcohol (V:V=24:1) extract , after gently mixing dozens of times, place on ice for 30min; centrifuge at 4°C, 10000r / min for 10min, take the supernatan...
Embodiment 3
[0068] Example 3: Determination of Primers ADZE / ADZF on the Minimum Detection Amount of Spiral Whitefly
[0069] 1) Preparation of spiral whitefly template
[0070] Single-headed spiral whitefly female adults were placed on a parafilm membrane dripped with 20 μL of extraction buffer (50 mmol / L Tris-HCl, 1 mmol / L EDTA, 1% SDS, 20 mmol / L NaCl, pH 8.0), and 0.2 mL of The bottom of the PCR tube was fully ground as a homogenizer, and the homogenate was transferred into a 1.5mL centrifuge tube with a micropipette; then the homogenizer was washed with 100 μL buffer solution four times, transferred to the same centrifuge tube, mixed evenly, and 5 μL of proteinase K (20 mg / mL), after fully mixing, place in a water bath at 60°C for 1 h (mix once in the middle); then bath in boiling water for 5 min, add 220 μL of chloroform / isoamyl alcohol (V:V=24:1) extract, and mix gently for several After ten times, place on ice for 30 minutes; centrifuge at 4°C, 10,000 r / min for 10 minutes, take th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com