Protein or polypeptide with function of CD137L, and gene and application thereof
A protein and functional technology, applied in the direction of peptides containing affinity tags, peptides containing His tags, medical preparations containing active ingredients, etc., can solve the problems of difficult industrialization, high cost, obvious side effects, etc. Purification, improved release, and the effect of short expression cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: Construction of recombinant protein gene expression vector with CD137L function
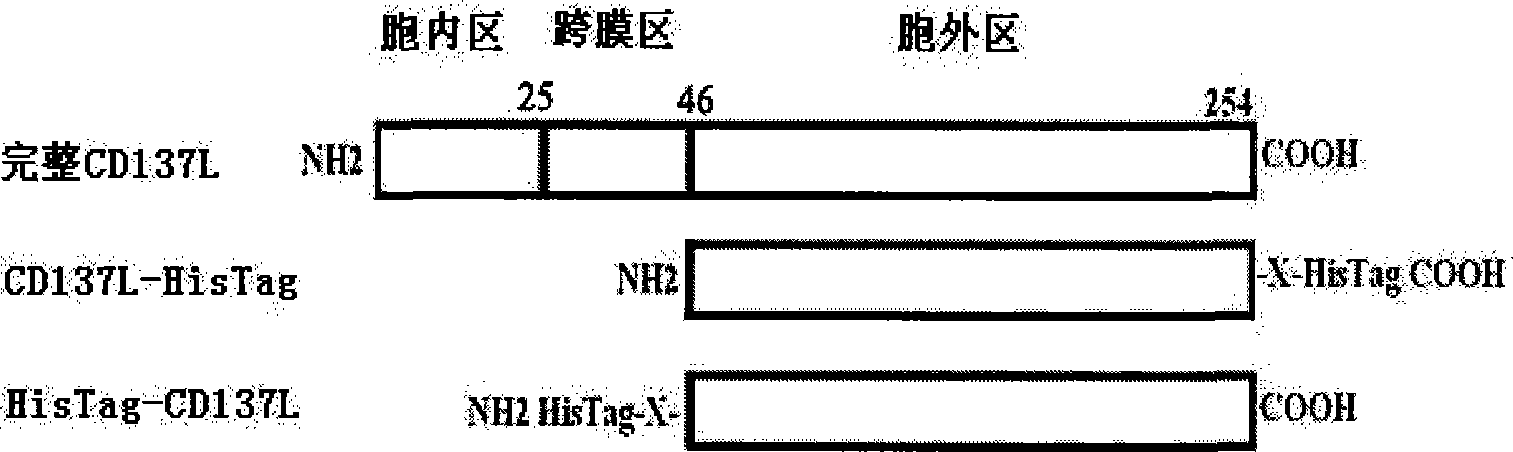
[0044] A total of 16 proteins or polypeptides with CD137L function were designed, the nucleotide sequences of which are SEQ ID NO.1-16, and the corresponding amino acid sequences are SEQ ID NO.17-32. The protein or polypeptide is a fusion of CD137L extracellular region protein and His-tag tag through a connecting peptide of 0-3 amino acids, preferably a connecting peptide of 1-3 amino acids, of which there are 4 kinds of His-tag tags at the N-terminus, There are 12 species at the C-terminus ( figure 1 ). The amino acid linking peptide can be one of the following amino acid combinations: Leu-Glu, Arg-Leu-Glu, Ile-Leu-Glu, Pro-Leu-Glu, Cys, Met, Tyr, Lys, Leu-Cys, Ile -Lys, Asp-Lys, Thr-Glu, Ile-Tyr-Met, Thr-Leu-Val and Asp-Ser-Lys.
[0045] The upstream primer of SEQ ID NO.1-4 is: 5'-TCATATG GCCGTCTTCCTCGCCT-3';
[0046] The downstream primers of SEQ ID NO.1-4 are respecti...
Embodiment 2
[0077] Example 2: Expression of recombinant protein with CD137L function
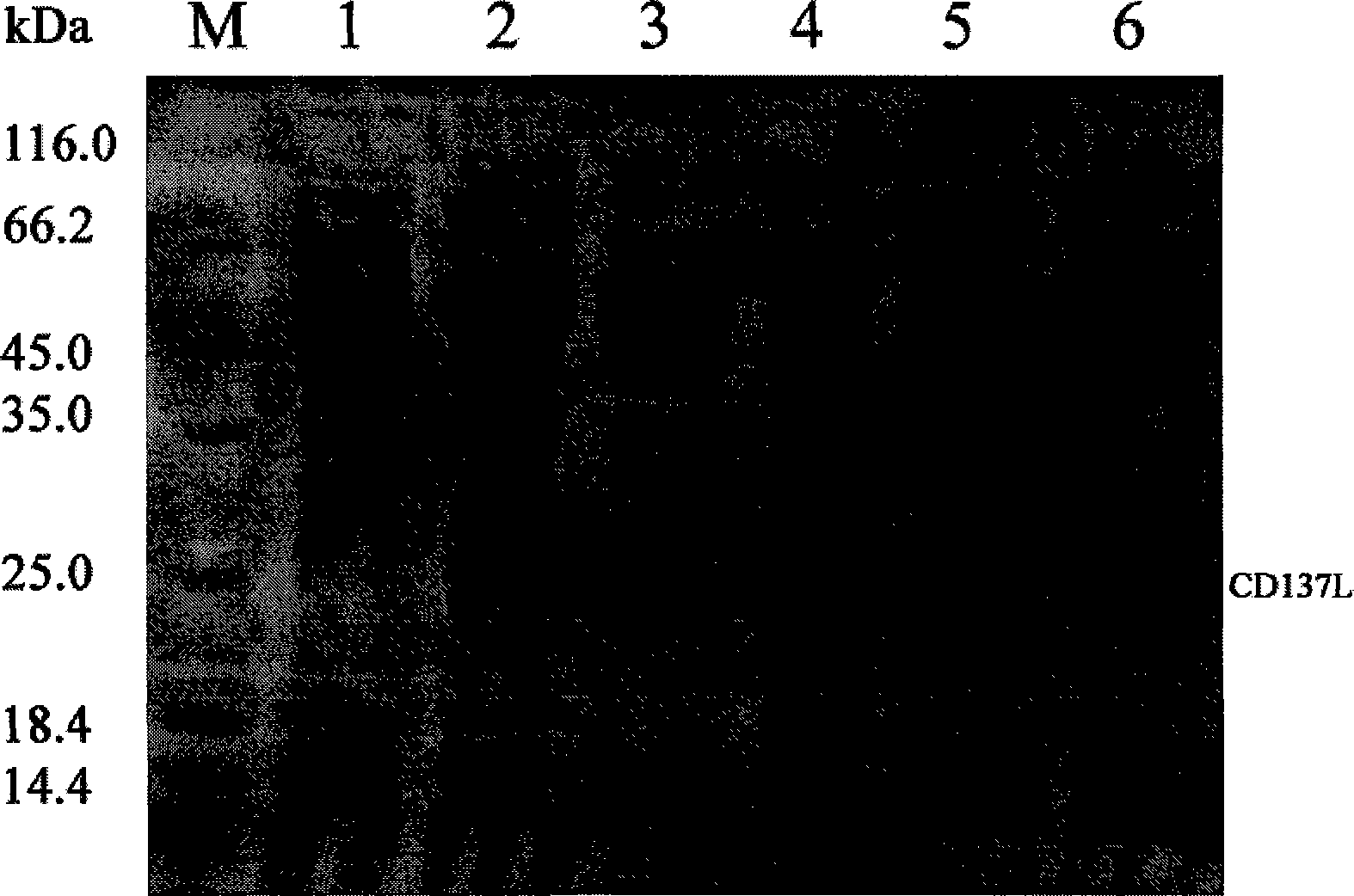
[0078] In an ultra-clean bench, add 1 mL of the Escherichia coli BL21 (DE3) strain containing the target gene plasmid to the LB medium containing 100 μg / mL ampicillin to 100 mL of the bacterial liquid, place on a shaker, 37 ° C, 250 rpm overnight culture under conditions. Transfer the activated strains to new sterilized LB medium, at OD 600 When the value is 0.9, add IPTG to make the final concentration of the same strain 0, 0.1, 0.2, 0.3, 0.4 and 0.5 mM respectively, cultivate at 15°C for 16 hours, and then centrifuge to collect the bacteria. Bacteria were suspended in 10 mL of PBS according to the wet weight of 1 g, added to a high-pressure cell breaker to break the cells, and the effluent was collected, centrifuged at 12,000 rpm at 4°C for 30 min, the precipitate was discarded, and the supernatant was retained for SDS-PAGE analysis.
[0079] The specific operation of SDS-PAGE is as follows:
[008...
Embodiment 3
[0085] Example 3: Purification of recombinant protein with CD137L function
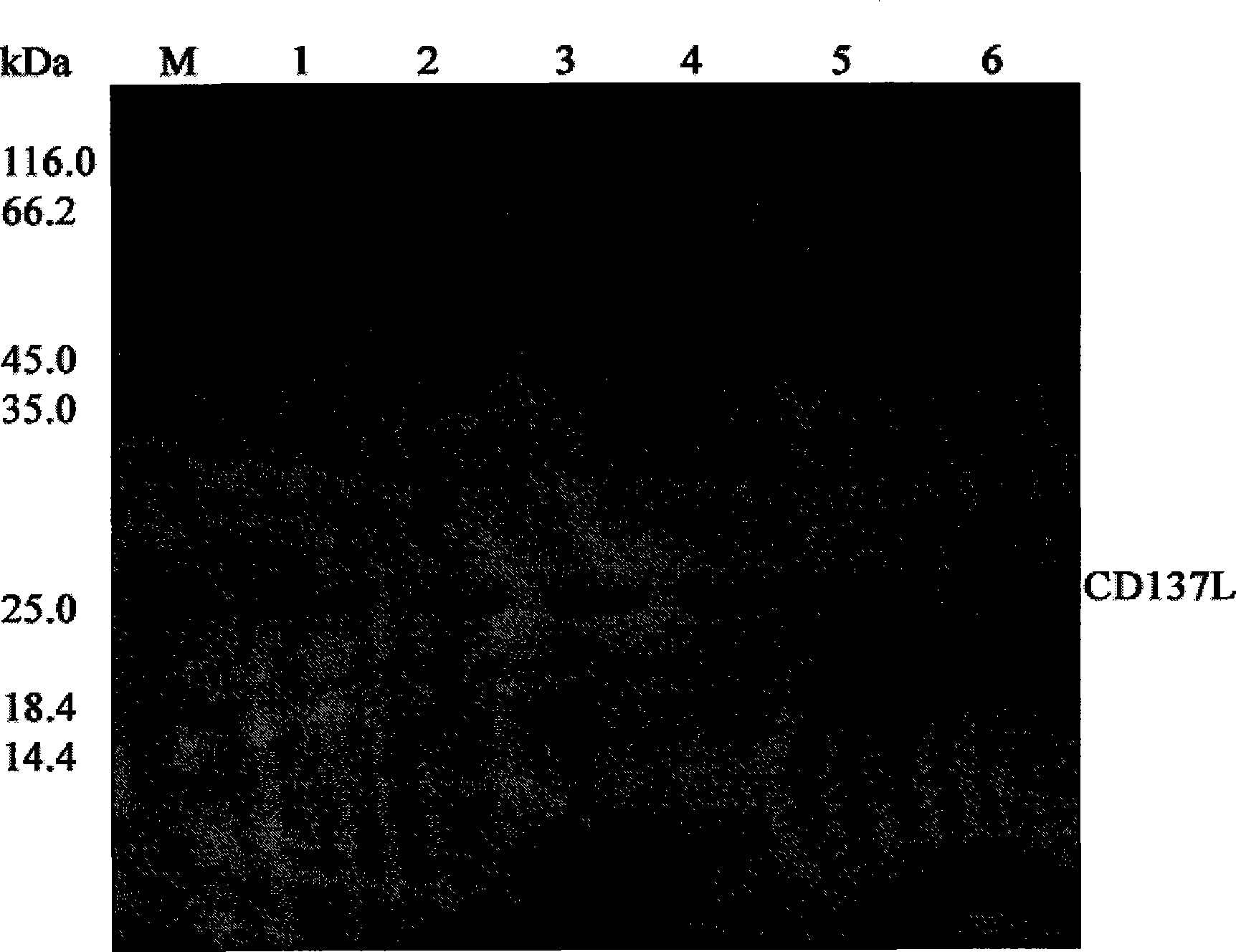
[0086] The supernatant obtained after high-pressure crushing was collected by the above method, and filtered with a 0.45 μm filter membrane. The target protein was purified by Ni Sepharose High Performance chromatographic column of GE Company, and the buffer solution containing 20mM, 50mM, 100mM and 400mM imidazole concentration was used for stage elution respectively, and the flow rate was 2ml / min, and the elution peaks of each stage were collected, SDS- The effect of protein purification was detected by PAGE. The experimental results showed that most of the miscellaneous proteins were gradually eluted with the increase of the imidazole concentration in the buffer. When the imidazole concentration rose to 50mM, the recombinant fusion protein began to be eluted; when the imidazole concentration rose to 100mM, some Miscellaneous proteins are eluted at the same time; and when the imidazole concentratio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com