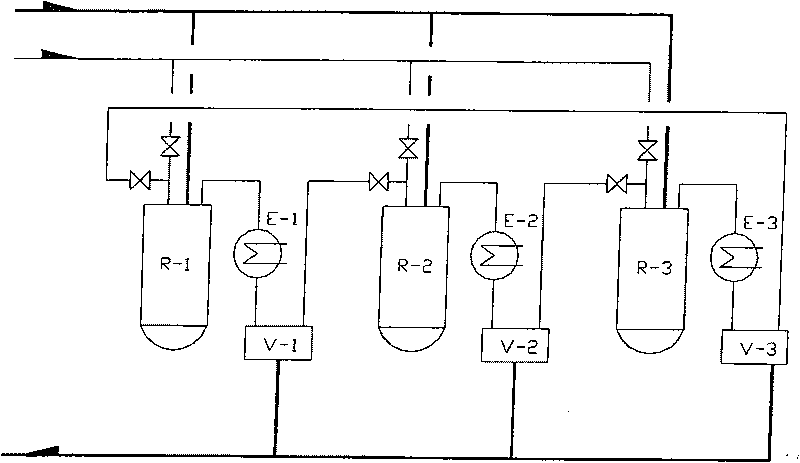
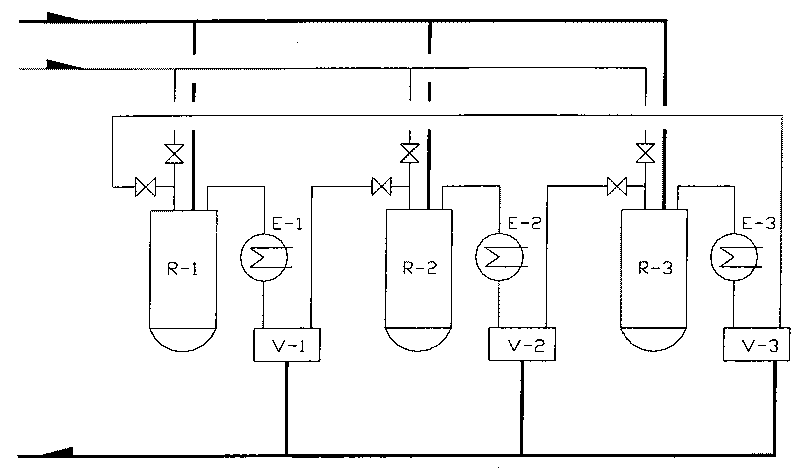
Method for synthesizing dichloropropanol by catalyzing glycerol for chlorination under existence of dicarboxylic acid-rare earth chloride
A rare earth chloride, dichloropropanol technology, applied in the direction of organic compound/hydride/coordination complex catalyst, physical/chemical process catalyst, chemical/physical process, etc., can solve the large loss of hydrogen chloride and increase the separation device , low utilization rate of equipment, etc., to achieve the effect of high activity, high reaction efficiency, and high utilization rate of hydrogen chloride
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]Add 650Kg glycerin (99.5%, W / W), 66Kg adipic acid (99%, W / W) and 0.65Kg cerium trichloride respectively in volume 1000L reactor R-1, R-2 and R-3, When the content of R-1 is heated to 100°C, hydrogen chloride gas is introduced under stirring, and at 100-120°C for 15 hours, a total of 1300Kg is introduced. After V-1 is received, hydrogen chloride tail gas is passed into R-2. After the chlorination reaction of R-1 is completed, dichloropropanol is distilled off under reduced pressure, and hydrogen chloride gas is passed into R-2 at the same time, and the generated water and part of dichloropropanol are Chloropropanol is stripped and distilled by hydrogen chloride. V-2 is received, and the hydrogen chloride tail gas of R-2 is passed into R-3. After R-1 dichloropropanol is evaporated under reduced pressure, add 500Kg glycerin and continue at 100-120°C Chlorination, start depressurizing to distill out the dichloropropanol of R-2 at this moment, feed hydrogen chloride gas to R-...
Embodiment 2
[0032] Equipment and method of operation are the same as in Example 1. 685Kg glycerol (95%, W / W), 66Kg adipic acid (99%, W / W) and 0.65Kg cerium trichloride were added. After ten cycles of production, the yield of dichloropropanol is 93% (calculated by the amount of glycerol).
Embodiment 3
[0034] Equipment and method of operation are the same as in Example 1. 650Kg glycerol (99.5%, W / W), 66Kg adipic acid (99%, W / W) and 0.65Kg lanthanum trichloride (99%, W / W) were added.
[0035] After ten cycles of production, the yield of dichloropropanol is 92% (calculated by the amount of glycerol).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com