Human alpha-defensin 5 antiviral active mutant polypeptide and preparation method and application
An antiviral activity and mutant technology, which is applied in the preparation methods of peptides, antiviral agents, botanical equipment and methods, etc., can solve the problems such as the decline of antibacterial and antiviral activities of defensins, and achieve the improvement of antiviral ability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1HD5
[0071] The solid-phase chemical method synthesis of embodiment 1HD5 mutant polypeptide:
[0072] 1. According to the amino acid sequence of the HD5 mutant, use a fully automatic peptide synthesizer (433A, Applied Biosystem) to entrust Shanghai Sangon Bioengineering Company to synthesize the HD5 mutant polypeptide.
[0073] 2. Purify the synthesized HD5 mutant polypeptide by HPLC reverse phase C18 column chromatography and desalting.
[0074] 3. Use the same method to synthesize and purify the maternal HD5 polypeptide reference substance.
[0075] 4. For the purified HD5 mutant polypeptide, use high pressure liquid chromatography (HPLC) method for purity identification, apply matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF) to determine its molecular weight, and determine its molecular weight by isoelectric focusing electrophoresis. The isoelectric point was determined by an automatic amino acid sequencer.
[0076] 5. The results of HPL...
Embodiment 2
[0077] Example 2 Expression and Purification of Recombinant HD5 Mutant Polypeptides in Pichia pastoris
[0078] 1. Synthesize the full-length gene encoding the HD5 mutant. Entrusted Shanghai Sangon Bioengineering Company to synthesize the full-length gene encoding the HD5 mutant, the gene sequence is as follows:
[0079] GCC ACC TGC TAT TGC CGA ACC GGC CGT TGT GCT ACC CGT GAG TCC CTC TCCGGG GTG TGT ATC AGT GGC CGC CTC TAC AGA CTC TGC TGT CGC
[0080] Note: The amino acid encoded by the 3 bases in the shaded part is the mutated arginine, and the 3 bases in the box encode the stop codon.
[0081] 2. Construction of recombinant HD5 mutant yeast expression vector
[0082] (1) PCR primer design: In order to ensure that there is no redundant amino acid at the N-terminal of the recombinant HD5 mutant expressed in yeast, the gene sequence encoding the HD5 mutant was directly inserted into the STE of the yeast expression vector pPIC9K using Red / ET recombination technology 13 aft...
Embodiment 3
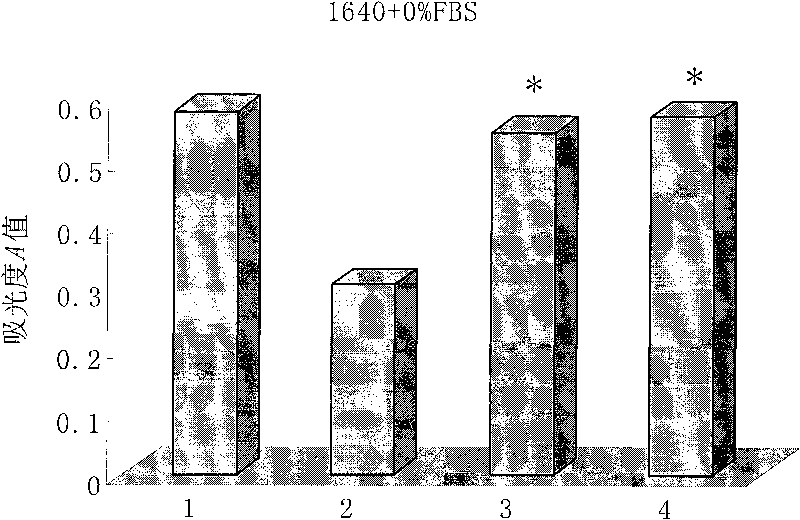
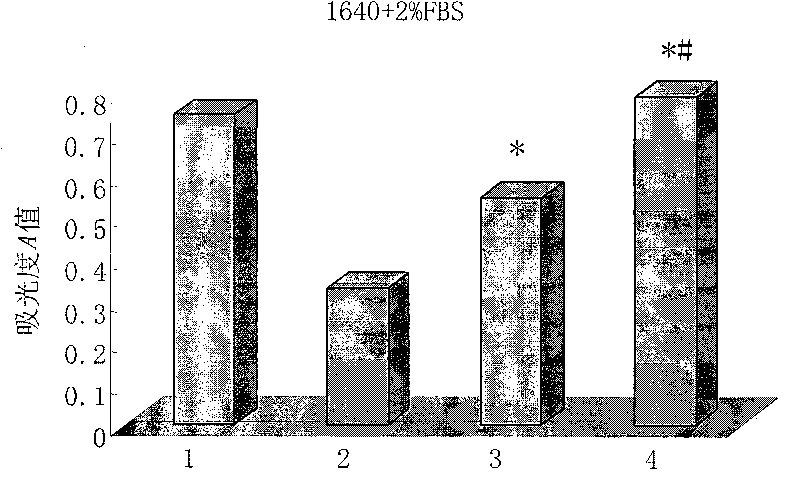
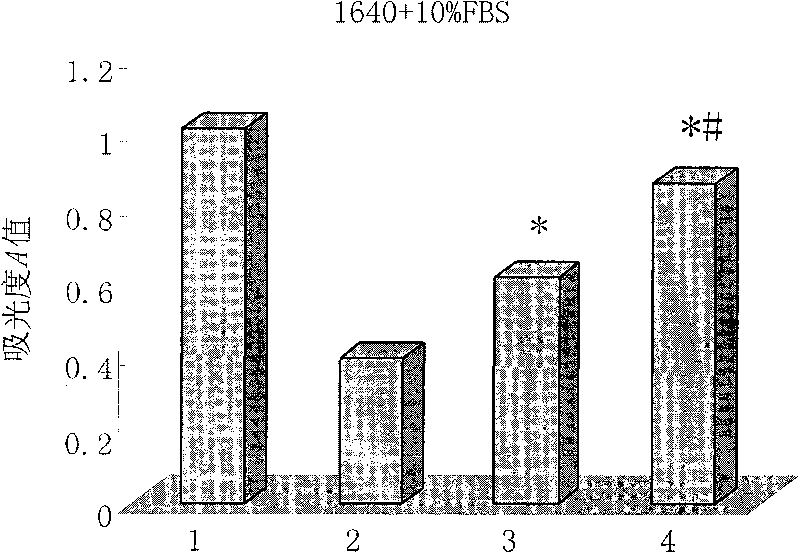
[0091] Cytotoxicity assay of embodiment 3HD5 mutant polypeptide
[0092] Inoculate vero cells (African green monkey kidney cells) in a 96-well plate with a seeding density of 2000 cells / well and a seeding volume of 100 μl. The vero cells were cultured in 1640 culture medium containing 10% fetal bovine serum for 24 hours until the cells were completely attached. The culture medium was discarded, and the culture medium containing different concentrations of HD5 mutant polypeptides was replaced to continue the culture. Each concentration was repeated for 5 wells, and a maternal HD5 polypeptide control group and a blank control group were set at the same time. After culturing for 72 hours, add 10 μl of CCK-8 reaction solution to each well, continue culturing at 37°C for 4 hours, discard the supernatant gently, shake and mix well, and measure the absorbance value (A value) at 490 nm wavelength with a microplate reader. Cell survival rate (%)=A value of the drug group / A value of the b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com