Vaccine eye drops used for treating and preventing glaucoma and preparation method
A technology for eye drops and glaucoma, applied in the field of vaccines, achieves low cost, promotes survival and regeneration, and is easy to accept
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
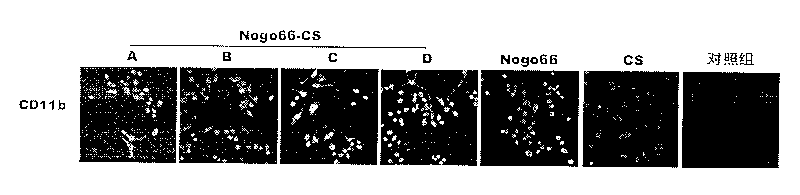
[0119] Preparation of Example 1 Nogo66-CS Vaccine Eye Drops
[0120] Under sterile conditions, take 1.5mg / ml Nogo66 protein solution (Xie Lin, He Xiangge, Su Yueyue, etc., "Prokaryotic expression and identification of Nogo-66 fusion protein", "Journal of Immunology", 2005, 21(3): 251 )0.1ml, 0.5ml, 1.0ml, 1.5ml, add 1.4ml, 1ml, 0.5ml, 0ml of double distilled water respectively, and make 0.1mg / ml, 0.5mg / ml, 1.0mg / ml, 1.5mg / ml Nogo66 protein solution. Take another 10mg, 50mg, 100mg, 150mg of chitosan, add 10ml of double distilled water respectively, drop an appropriate amount of acetic acid to dissolve it completely, and prepare 1mg / ml, 5mg / ml, 10mg / ml, 15mg / ml chitosan solutions . The above-mentioned Nogo66 protein solution and chitosan solution were filtered 3-5 times with a 0.22 μm sterile microporous filter, respectively, and sterilized.
[0121] Mix the above-mentioned 0.1mg / ml Nogo66 protein solution and 1mg / ml chitosan solution in equal volumes after sterilization, sti...
Embodiment 2
[0127] Example 2 General inspection
[0128] 1. Properties: light yellow, slightly viscous and clear liquid
[0129] 2. PH value: The pH values of A, B, C, and DNogo66-CS vaccine eye drops samples measured by a pH detector were 7.014, 7.127, 7.046, and 7.068, respectively.
[0130] 3. Osmotic pressure: Take 3 samples of Nogo66-CS vaccine eye drops of 4 concentrations and 3 samples of 0.9% NaCl solution, respectively, and test the osmotic pressure respectively. Results A, B, C, D The average osmotic pressure of Nogo66-CS vaccine eye drops samples were: 285mosm / L, 286mosm / L, 291mosm / L, 299mosm / L, and the average osmotic pressure of 0.9% NaCl solution samples was 301mosm / L . A, B, C, D The osmotic pressure of Nogo66-CS vaccine eye drops is equivalent to 0.95-0.99 times the osmotic pressure of 0.9% NaCl solution, which meets the requirements of eye drop preparations.
[0131] 4. Content determination: Precisely prepare bovine serum albumin solutions with concentrations of 0.2...
Embodiment 3
[0133] Example 3 Nogo66-CS vaccine eye solution acute eye irritation, corrosion test
[0134] Select 10 healthy adult SD rats with normal eyes and corneal fluorescein examination. Rats were anesthetized by intraperitoneal injection of 3% pentobarbital sodium at 1.3ml / kg. After the anesthesia was complete, the head of the animal was tilted to the left, the right eye was obliquely upward, and the double eyelids were gently lifted. A, B, C, and D Nogo66 -CS was instilled on the cornea of the right eye with 0.05ml, once / 10min, and lasted for 1 hour for a total of 6 times; at the same time, the left eye was given normal saline with the same strategy as the control eye. Observation at the time of drug application and 1h, 24h, 48h, 72h and the 4th and 7th day after drug application: tears and secretions, conjunctival congestion and edema, conjunctival follicular papilla, corneal edema and turbidity, corneal punctate epithelial damage, Iris damage. And score according to the eye i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com