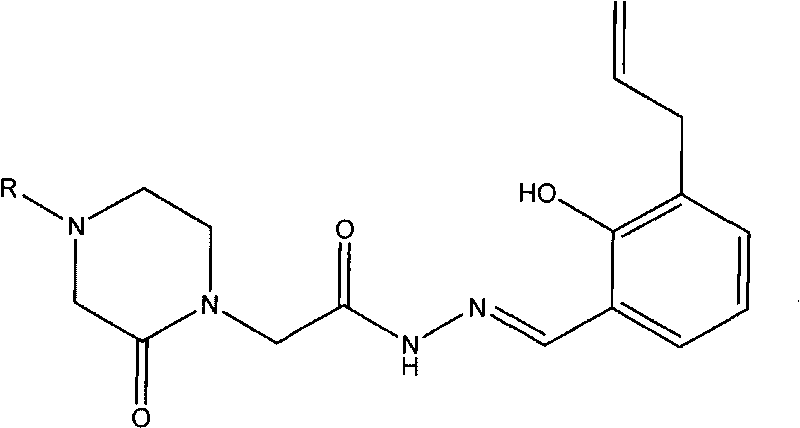
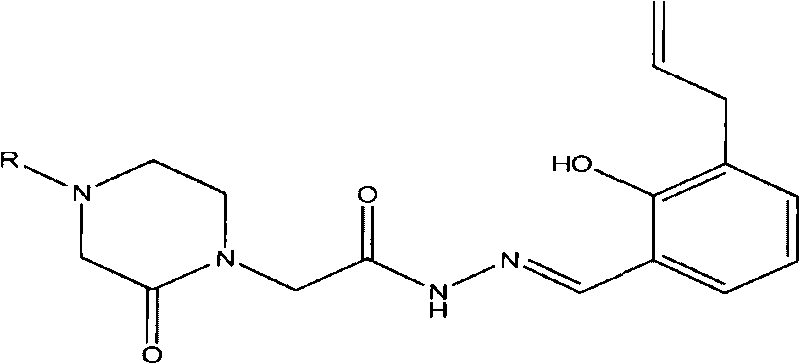
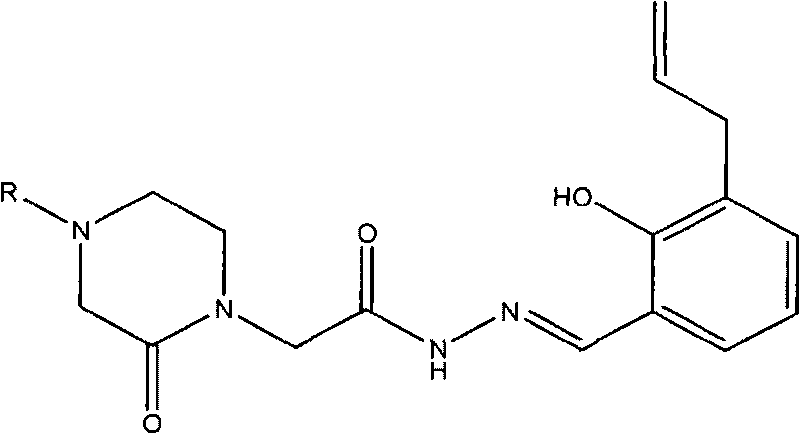
Acetamide derivative and application thereof in pharmacy
A technology of acetamide and derivatives, applied in the field of medicine, can solve the problems of listing, no drugs, and cancer cells initiating programmed death.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1.2-(4-benzyl-2-piperazinon-1-yl)-N-[(2-hydroxyl-3-allyl-phenyl)methyleneamino]acetamide (I 1 ) preparation and structure confirmation
[0037] (1). Synthesis of 4-benzyl-2-piperazinone
[0038] Add 5.1g of 2-piperazinone, 5.0g of benzyl chloride and 4.2g of sodium bicarbonate into 60ml of ethanol respectively, stir, heat to reflux for 5h, cool to room temperature, filter, concentrate the filtrate to obtain a solid, recrystallize from benzene to obtain 8.6g of white crystals (melting point 152-154°C).
[0039] (2). Synthesis of 2-(4-benzyl-2-piperazinon-1-yl)-ethyl acetate
[0040] Add 8.55g of 4-benzyl-2-piperazinone and an appropriate amount of sodium hydride into dry 100mL of acetone, stir, add 6.2g of ethyl bromoacetate dropwise, start heating and refluxing until the reaction of the raw materials is complete, cool, filter, After washing, the filtrate was concentrated to obtain an oil, and column chromatography (eluent: ethyl acetate / n-hexane=4 / 1) obtained...
Embodiment 2
[0047] Example 2.2-[4-(3-pyridyl-methyl)-2-piperazinon-1-yl]-N-[(2-hydroxyl-3-allyl-phenyl)methyleneamino ] Acetamide (I 2 ) preparation and structure confirmation
[0048] (1). Synthesis of 4-(3-pyridyl-methyl)-2-piperazinone
[0049] Add 5.1g 2-piperazinone, 5.0g 3-chloromethylpyridine and 4.2g sodium bicarbonate into 60ml of ethanol respectively, stir, heat to reflux for 5h, cool to room temperature, filter, concentrate the filtrate, column chromatography (eluent : chloroform / methanol=10 / 1), collect eluent, concentrate to obtain product.
[0050] The resulting product obtains the target product in the same manner as (2), (3), and (4) in Example 1.
[0051] 1 H-NMR (DMSO, -d 6 )δ8.75(s, 1H), 8.6(s, 1H), 8.15(s, 1H), 7.95(s, 1H), 7.8(s, 1H), 7.5(s, 1H), 7.2(s, 1H ), 6.8(s, H), 6.65(s, H), 6.25(s, H), 5.16(s, 1H), 4.91(ddd, 2H), 4.12(dd, 2H), 3.66(dd, 2H) , 3.37(s, 2H), 3.24(d, 2H), 3.16(d, 2H), 2.64(d, 2H).
[0052]
Embodiment 3
[0053] Example 3.2-(4-p-toluenesulfonyl-2-piperazinonyl-1-yl)-N-[(2-hydroxyl-3-allyl-phenyl)methyleneamino]acetamide (I 3 ) preparation and structure confirmation
[0054] (1). Synthesis of 4-p-toluenesulfonyl-2-piperazinone
[0055] Add 5.1g of 2-piperazinone, 7.6g of p-toluenesulfonyl chloride and 5.1g of triethylamine into 60ml of dichloromethane respectively, stir, heat to reflux for 5h until the reaction is complete, cool to room temperature, filter, concentrate the filtrate, and perform column chromatography (Eluent: chloroform / methanol=10 / 1), collect the eluent, and concentrate to obtain the product.
[0056] The resulting product obtains the target product in the same manner as (2), (3), and (4) in Example 1.
[0057] 1 H-NMR (DMSO-d 6 )δ8.15(s, 1H), 7.90(s, 1H), 7.81(s, 2H), 7.35(s, 1H), 7.23(s, 2H), 6.9(s, H), 6.63(s, H ), 6.2(s, H), 5.10(s, 1H), 4.91(ddd, 2H), 4.2(dd, 2H), 3.38(s, 2H), 3.28(d, 2H), 3.16(d, 2H) , 2.60(d, 2H), 2.32(d, 3H).
[0058]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com