Method for detecting release of anaphylactic medium in Chinese medicament injection
An injection and allergy technology, which is applied in the field of rapid detection of the release of allergic mediators caused by traditional Chinese medicine injections, and the detection of endogenous trace substances, can solve the problems of low specificity of histamine, cumbersome operation steps, and difficulty in accurate quantification.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 1. Preparation of reaction solution
[0031] Prepared by mixing 1110 μL of absolute ethanol, 240 μL of triethylamine, 50 μL of 2 μg / mL 9-methylanthracene, and 3.6 mL of acetone;
[0032] 2. Preparation of derivatization solution
[0033] It is composed of derivatization reagent Ethylchloroformate (ethyl chloroformate) mixed with chloroform in a certain proportion, specifically ethyl chloroformate and chloroform in a volume ratio of 1:9;
[0034] 3. Derivatization preparation of the test solution
[0035] Add 1350 μL of reaction solution to 100 μL of plasma or cell culture supernatant, vortex on a vortex for 3 seconds, add 550 μL of derivatization solution, and continuously vortex for 10 seconds; add 3.5 mL of water and vortex for 5 seconds; centrifuge at 1500 rpm for 5 minutes , take the organic phase and put it in a clean bottle, add 10 mg of anhydrous sodium sulfate for dehydration, and place it in a GC automatic sampling bottle with a liner for analysis.
[0036] ...
Embodiment 2
[0044] 1. Preparation of reaction solution
[0045] It is prepared by mixing 1000 μL of absolute ethanol, 200 μL of triethylamine, 50 μL of 2 μg / mL 9-methylanthracene, and 2.5 mL of acetone; that is, absolute ethanol: triethylamine: 9-methylanthracene solution: acetone = 30:6: 1:80~20:4:1:50,
[0046] 2. Preparation of derivatization solution
[0047] It is composed of derivatization reagent Ethylchloroformate (ethyl chloroformate) which is miscible with chloroform in a certain proportion, specifically ethyl chloroformate and chloroform in a volume ratio of 2:8;
[0048] 3. Derivatization preparation of the test solution
[0049] Add 1350 μL of reaction solution to 100 μL of plasma or cell culture supernatant, vortex on a vortex for 3 seconds, add 550 μL of derivatization solution, and continuously vortex for 10 seconds; add 3.5 mL of water and vortex for 5 seconds; centrifuge at 1500 rpm for 5 minutes , take the organic phase and put it in a clean bottle, add 10 mg of anhy...
Embodiment 3
[0051] 1. Linear relationship inspection
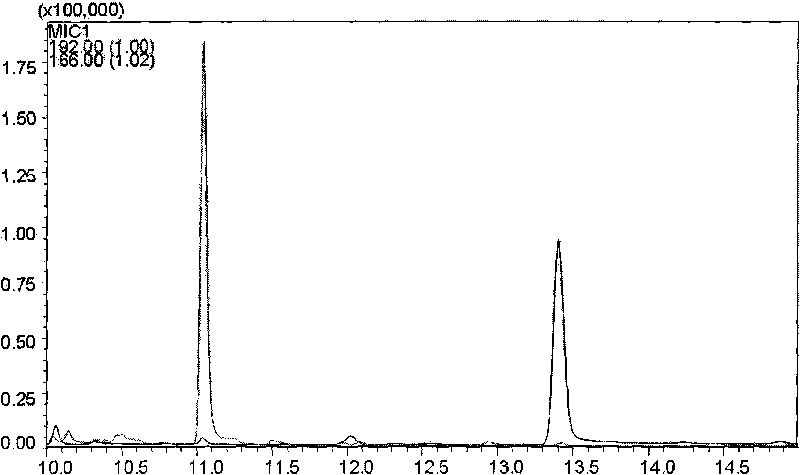
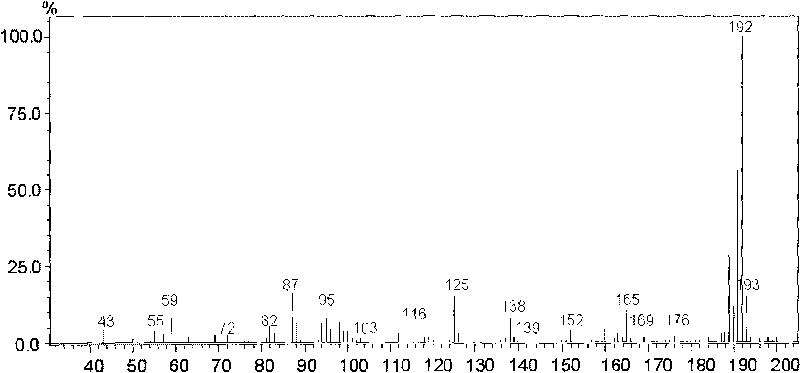
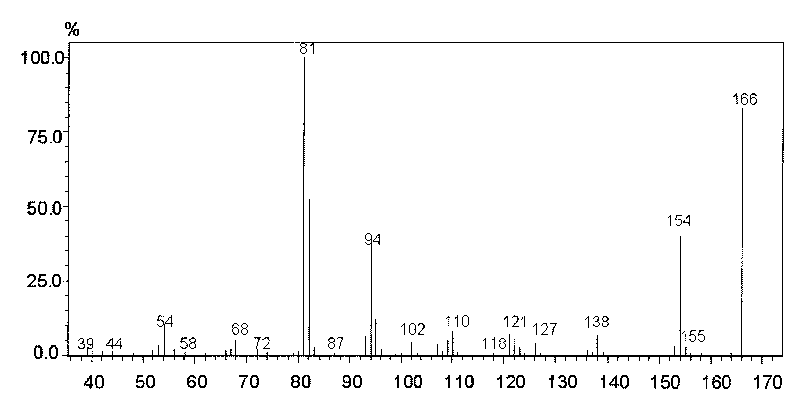
[0052] The linear relationship is to investigate the degree to which the change of the concentration of the tested sample is directly proportional to the result of the sample, and it is often determined by drawing a standard curve. Accurately draw 0.01, 0.1, 0.25, 0.5, 1, 2.5ml of histamine reference substance stock solution (2μg / mL) respectively and put them in a 10ml measuring bottle. , the method under the three items is measured, take reference substance concentration as abscissa, reference substance and internal standard substance peak area ratio as ordinate, draw standard curve, carry out regression analysis, get regression equation Y=1.0726X+0.031, r=0.9993 , indicating that histamine has a good linear relationship in the range of 0.002-2 μg / ml. see Figure 4 .
[0053] 2. Repetitive experiments
[0054] Take the same solution of the test product, repeat the sample injection 5 times according to the method under item 2 and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com