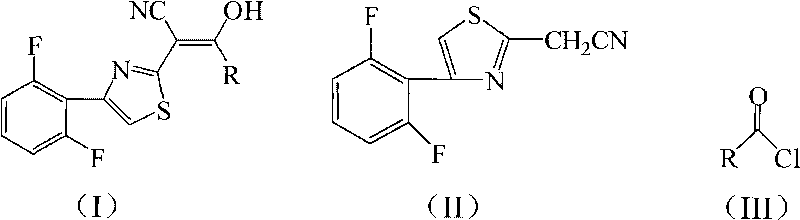
2-thiazolyl acrylonitrile compounds and synthesis and application thereof
A technology of thiazolyl acrylonitrile and compound is applied in the field of 2-thiazolyl acrylonitrile compounds and their synthesis, and can solve the problem that 2-thiazolyl acrylonitrile compounds are not widely used and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
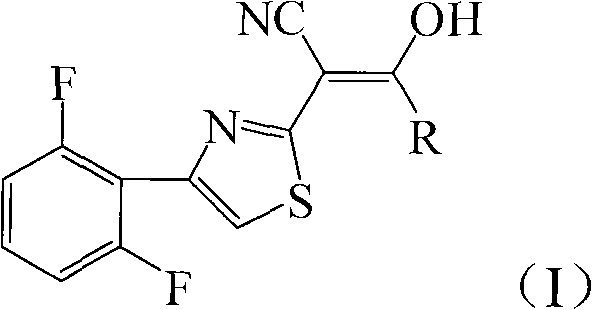
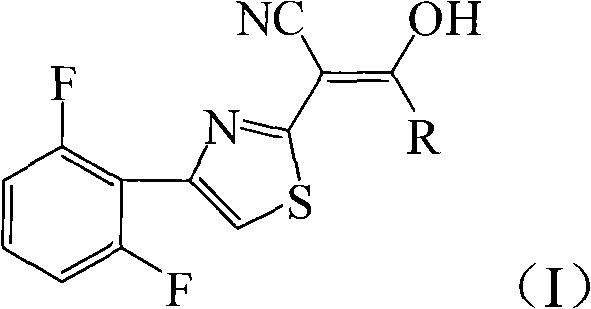
[0026] Add 4.72g (20mmol) 2-cyanomethyl-4-(2,6-difluorophenyl) thiazole and 3.05g (30mmol) triethylamine to 20mL tetrahydrofuran, add dropwise 2.76g (24mmol) chloroacetyl chloride , 40min dripping, then reacted at 66°C for 12h, after the reaction was completed, filtered, and the filter cake was recrystallized with chloroform to obtain light green crystal 2-[4-(2,6-difluorophenyl)-2-thiazolyl]- 4.40g of 3-hydroxy-3-chloromethylacrylonitrile, melting point 193-195°C, yield 70%.
[0027] of the compound 1 HNMR and IR are as follows,
[0028] 1 HNMR (CDCl 3 )δ=4.43(H, s, CH 2 Cl), 7.09~7.13 (2H, m, Ar- CH -CH- CH ), 7.40 (H, s, Triazole), 7.42~7.50 (H, m, Ar-CH- CH -CH), 14.67 (H, OH) IR (KBr, cm -1 )3378, 3155, 2201, 1524, 1610~1450, 1233, 783, 727
Embodiment 2
[0030] 4.72g (20mmol) 2-cyanomethyl-4-(2,6-difluorophenyl) thiazole and 1.13g (28mmol) sodium hydroxide were added to 25mL 1,4-dioxane, and 2.10g ( 26mmol) of acetyl chloride, dripping for 40min, then reacted at 100°C for 6h, after the reaction was completed, filtered, and the filter cake was recrystallized with chloroform to obtain white flaky crystals 2-[4-(2,6-difluorophenyl)-2 -Thiazolyl]-3-hydroxy-3-methacrylonitrile 4.36g, melting point 197-198°C, yield 78%.
[0031] of the compound 1 HNMR and IR are as follows,
[0032] 1 HNMR (CDCl 3 )δ=2.41(3H, s, CH 3 ), 7.05~7.09 (2H, m, Ar- CH -CH- CH ), 7.36~7.46 (2H, m, Ar-CH- CH -CH& Triazole)
[0033] IR (KBr, cm -1 )3453, 3179, 2191, 1513, 1610~1450, 1378, 1246, 790, 712
Embodiment 3
[0035] Add 4.72g (20mmol) 2-cyanomethyl-4-(2,6-difluorophenyl) thiazole and 3.22g (30mmol) sodium carbonate to 20mL dichloromethane, add dropwise 2.27g (24mmol) propionyl chloride , 40min dripping, and then reacted at 40°C for 12h, after the reaction was completed, filtered, and the filter cake was recrystallized with chloroform to obtain a light green solid 2-[4-(2,6-difluorophenyl)-2-thiazolyl]- 4.21 g of 3-hydroxy-3-ethylacrylonitrile, melting point 172-174°C, yield 72%.
[0036] of the compound 1 HNMR and IR are as follows,
[0037] 1 HNMR (CDCl 3 ) δ = 1.25 ~ 1.28 (3H, t, CH 3 ), 2.70~2.75 (2H, m, CH 2 ), 7.05~7.09 (2H, t, Ar- CH -CH- CH ), 7.37~7.41 (2H, m, Ar-CH- CH -CH& Triazole), 14.67 (H, OH)
[0038] IR (KBr, cm -1 )3450, 3161, 2194, 1513, 1620~1480, 1459, 1237, 997
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com