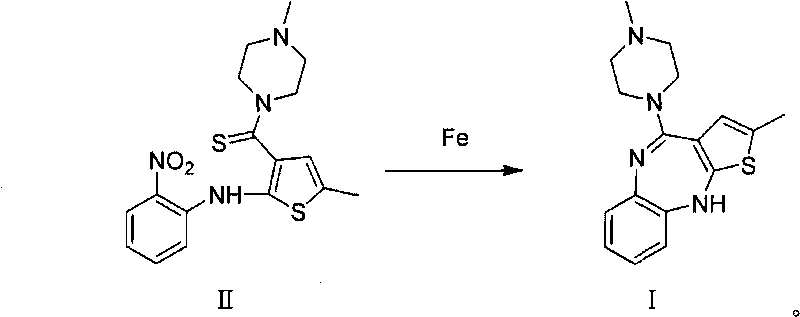
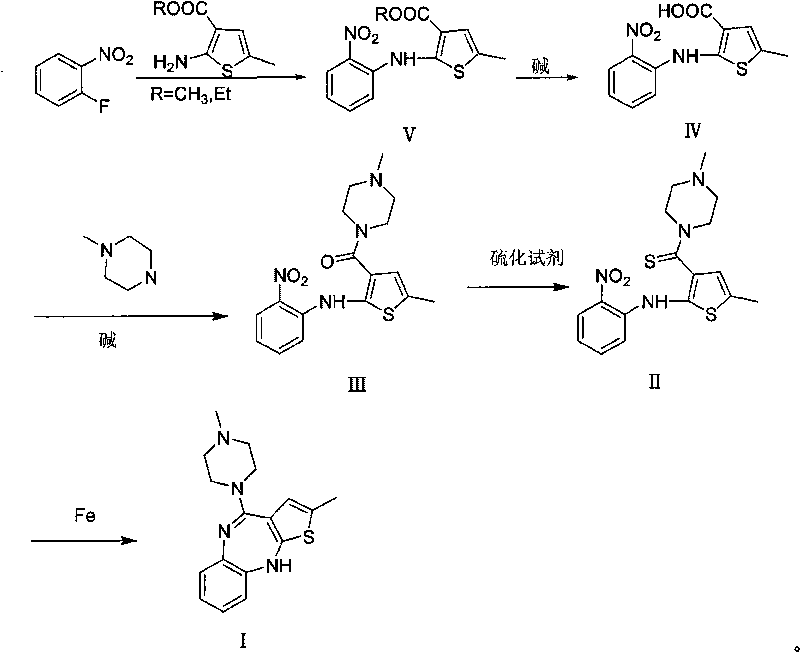
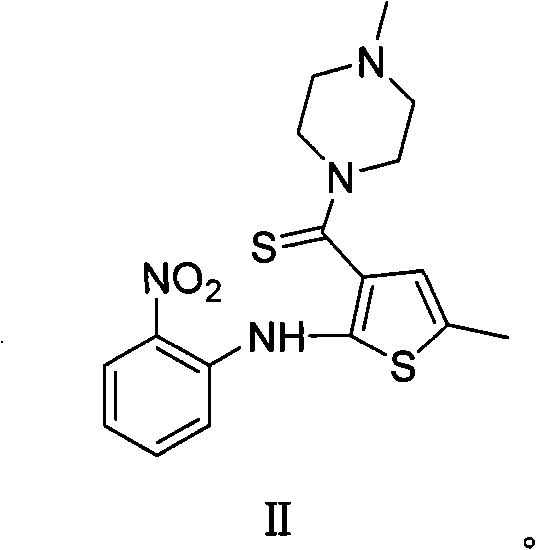
Technique for preparing olanzapine
A technology of methyl and nitroaniline, which is applied in the field of synthetic routes for the preparation of olanzapine, can solve the problems of low yield and low quality of the final product, achieve high yield, avoid highly toxic heavy metals, and easily control the reaction Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Synthesis of 5-methyl-2-(2-nitroanilino)-3-carboxymethylthiophene V:
[0039] Add 60mL of dimethyl sulfoxide, 5.5g of anhydrous potassium carbonate, 7.1g of o-fluoronitrobenzene, 6g of 2-amino-5-methyl-3-formic acid methyl thiophene into a 100mL three-necked flask, and heat under nitrogen protection React at 60-75°C for 20-30 hours until the reaction of the raw materials is complete, cool to room temperature, pour into 200mL ice water, precipitate solid, filter, wash with water, and recrystallize with ethanol to obtain 6 grams of 5-methyl-2-(2-nitro Anilino)-3-carboxymethylthiophene V (yield: 56%). 1 H NMR (400MHz, CDCl 3 )δ11.88(s, 1H), 8.26(d, J=8.4Hz, 1H), 7.90(d, J=8.4Hz, 1H), 7.60(t, J=7.4Hz, 1H), 7.00(t, J=7.4Hz, 1H), 6.92(s, 1H), 3.92(s, 3H), 2.40(s, 3H); UV: 329.6, 224.8, 193.8nm.
Embodiment 2
[0041] Synthesis of 5-methyl-2-(2-nitroanilino)-3-carboxythiophene IV:
[0042] Add 5.4 g of 5-methyl-2-(2-nitroanilino)-3-methylthiophene V and 100 mL of ethanol into a 250 mL three-necked flask, add 20 mL of 1.75 N aqueous sodium hydroxide solution dropwise under stirring, and the addition is complete Raise the temperature to 40-55°C and react for 3-5 hours until the reaction of 5-methyl-2-(2-nitroanilino)-3-methylthiophene V is complete. Cool to room temperature, add 50 mL of ice water, adjust pH=1-2 with 6N hydrochloric acid, filter, wash with water, and dry to obtain 4 g of red solid 5-methyl-2-(2-nitrophenylamino)-3-carboxythiophene IV ( Yield: 82%). 1 HNMR (400MHz, CDCl 3 )δ12.89(s, 1H), 11.73(s, 1H), 8.22(d, J=8.4Hz, 1H), 7.89(d, J=8.4Hz, 1H), 7.77(t, J=7.4Hz, 1H), 7.14(t, J=7.6Hz, 1H), 6.91(s, 1H), 2.37(s, 3H); 13 CNMR (100MHz, CDCl 3 ): 165.8, 150.3, 137.7, 136.8, 135.7, 127.1, 125.5, 124.0, 121.4, 117.3, 114.7, 14.9; MS: 278 (M + ), 277 (M + -1); IR(film, cm ...
Embodiment 3
[0044] Synthesis of 5-methyl-2-(2-nitroanilino)-3-(4-methylpiperazinyl)carbonylthiophene III:
[0045] Add 4g of 5-methyl-2-(2-nitroanilino)-3-carboxythiophene IV into a 100mL three-necked flask, dissolve 20mL of thionyl chloride in 20mL of toluene, heat to 42°C for reaction, and maintain the reaction temperature to The raw materials were completely reacted, concentrated under reduced pressure to remove thionyl chloride, added 28 mL of acetonitrile to dissolve, added dropwise 8 mL of N-methylpiperazine, poured into ice water after the reaction was completed, extracted with ethyl acetate, combined the organic phases, and concentrated to obtain 4.52 g of III (Yield: 90%). 1 H NMR (400MHz, CDCl 3 )δ10.17(s, 1H), 8.17(d, J=8.7Hz, 1H), 7.39-7.49(m, 2H), 6.86(t, J=8.4Hz, 1H), 6.62(s, 1H), 3.61(s, 4H), 2.44(s, 3H), 2.35(t, J=4.8Hz, 4H), 2.03(s, 3H); 13 C NMR (100MHz, CDCl 3 ): 164.6, 141.6, 139.6, 135.8, 133.6, 133.3, 126.7, 126.3, 123.1, 118.7, 116.1, 54.8, 45.9, 15.3; MS: 361 (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com