Oxiracetam liposome injection
A body injection and injection technology, applied in the field of medicine, can solve the problems of encapsulated drug leakage, easy aggregation, fusion, etc., and achieve the effect of small side effects, good solubility, and fast dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
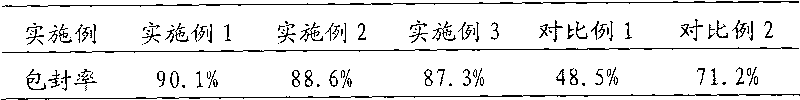
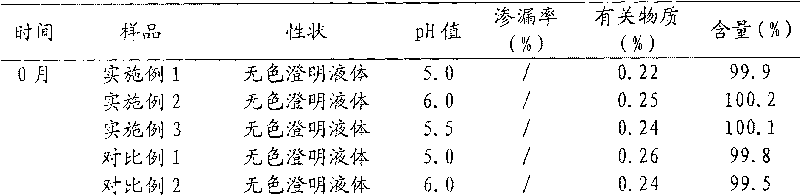
[0051] Example 1 Preparation of Oxiracetam Liposomal Injection
[0052] prescription:
[0053] Oxiracetam 100g
[0054] Soy Lecithin 480g
[0055] Cholesterol 320g
[0056] Tween 80 130g
[0058] Phosphate buffer solution 4000g
[0059] Preparation Process:
[0060] (1) Dissolve 480g soybean lecithin, 320g cholesterol and 130g Tween 80 in a mixed solvent of acetone and methanol in a volume ratio of 5000ml of 4:1, mix well, remove the mixed solvent under reduced pressure on a rotary thin film evaporator, and obtain Phospholipid membrane;
[0061] (2) Add 4000 g of disodium hydrogen phosphate-sodium dihydrogen phosphate buffer solution with a pH value of 5.0 to the prepared phospholipid film, shake, stir for 30 min, and rotate at 800 r / min to fully hydrate the phospholipid film, and mash it with tissue Machine high-speed homogeneous emulsification 20min, rotating speed is 15000r / min, 0.45μm microporous membrane filtration, makes blank lipo...
Embodiment 2
[0063] Example 2 Preparation of Oxiracetam Liposomal Injection
[0064] prescription:
[0065] Oxiracetam 100g
[0067] Cholesterol 120g
[0068] Tween 80 80g
[0069] Glucose 250g
[0070] Citrate buffer solution 4200g
[0071] Preparation Process:
[0072] (1) Dissolve 350g egg yolk lecithin, 120g cholesterol and 80g Tween 80 in a mixed solvent of acetone and methanol in a volume ratio of 3000ml of 4:1, mix well, remove the mixed solvent under reduced pressure on a rotary thin film evaporator, and obtain Phospholipid membrane;
[0073] (2) Add 4200 g of citric acid-sodium citrate buffer solution with a pH value of 6.0 to the prepared phospholipid film, shake, stir for 60 min, and rotate at 400 r / min to completely hydrate the phospholipid film, and use a tissue masher High-speed homogeneous emulsification for 40 minutes, the rotating speed is 15000r / min, and 0.45 μm microporous membrane filtration to obtain a blank liposome suspensi...
Embodiment 3
[0075] Example 3 Preparation of Oxiracetam Liposomal Injection
[0076] prescription:
[0077] Oxiracetam 100g
[0078] Hydrogenated egg yolk lecithin 700g
[0079] Cholesterol 500g
[0080] Tween 80 300g
[0081] Glycerin 500g
[0082] Citrate buffer solution 4000g
[0083] Preparation Process:
[0084] (1) 700g hydrogenated egg yolk lecithin, 500g cholesterol and 300g Tween 80 are dissolved in the mixed solvent of acetone and methanol of 12000ml volume ratio 4: 1, mix well, remove the mixed solvent under reduced pressure on the rotary thin film evaporator, prepare Get phospholipid membrane;
[0085] (2) Add 4000 g of citric acid-sodium citrate buffer solution with a pH value of 5.5 to the prepared phospholipid film, shake, stir for 40 min, and rotate at 600 r / min to completely hydrate the phospholipid film, and use a tissue masher High-speed homogeneous emulsification for 30 minutes, the rotation speed is 15000r / min, and 0.45 μm microporous membrane filtration to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com