Method for preparing aluminum oxide from aluminiferous material
A technology of alumina and materials, applied in the preparation of aluminum hydroxide, chemical instruments and methods, preparation of alumina/hydroxide, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] The main composition of the fly ash used is: Al 2 o 3 41.2%, SiO 2 48.5%, Fe 2 o 3 3.4%, CaO 3.3%, TiO 2 1.3%, MgO 0.2%.
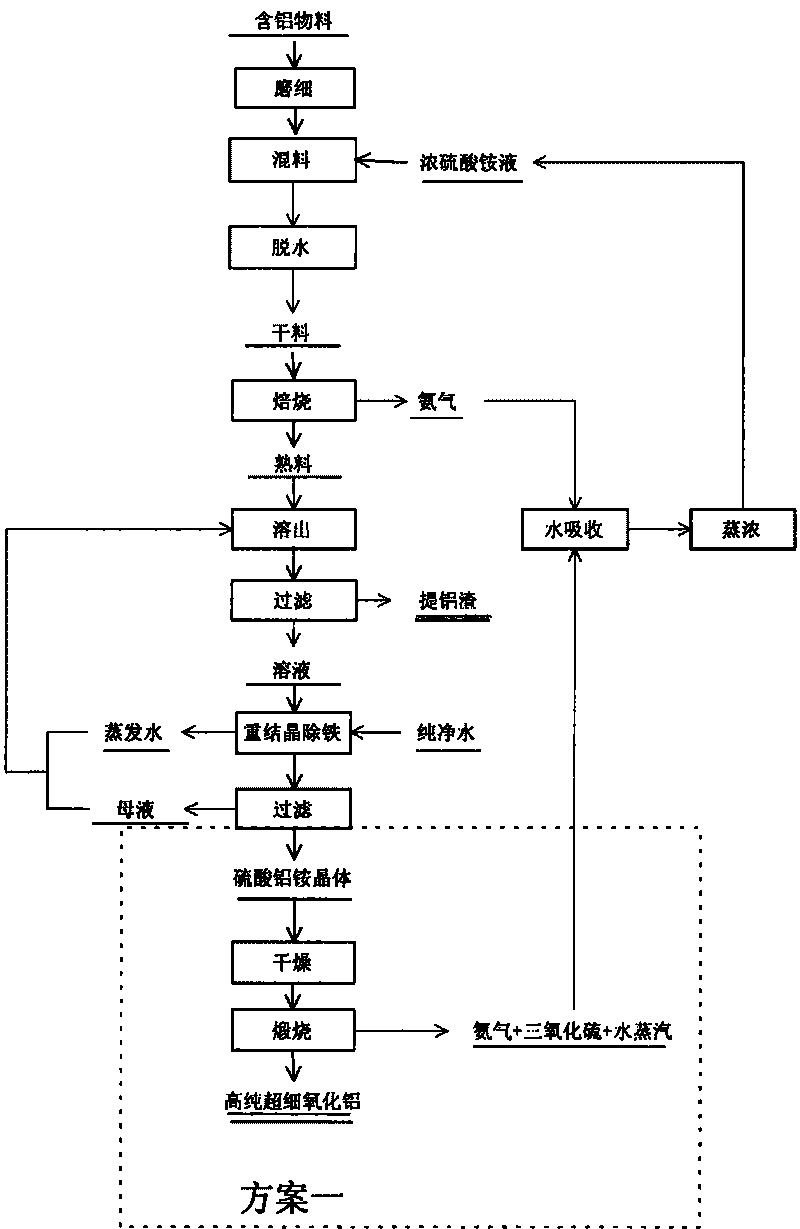
[0088] After the fly ash that has been crushed and ground to less than 80 μm is removed by magnetic separation, it is mixed with ammonium sulfate and water in a mass ratio of 1:3.2:5 (the molar ratio of alumina and ammonium sulfate is 1:6), and the ℃ roasting reaction for 5h, and absorb the ammonia gas generated during the reaction with water. The roasted product is lowered in temperature, dissolved by adding water, and then separated into solid and liquid. The filtrate is aluminum ammonium sulfate solution, and the filter residue is aluminum extraction slag.
[0089] After the ammonium aluminum sulfate solution is purified by recrystallization to remove impurities such as iron, pure ammonium aluminum sulfate crystals are prepared. Aluminum ammonium sulfate crystals are dehydrated at 200°C for 3 hours, decomposed at 550°C for 3 hours, an...
Embodiment 2
[0092] The composition of the bauxite used is: Al 2 o 3 65.3%, SiO 2 23.1%, Fe 2 o 3 4.8%, CaO 1.6%, TiO 2 3.5%, K 2 O0.5%.
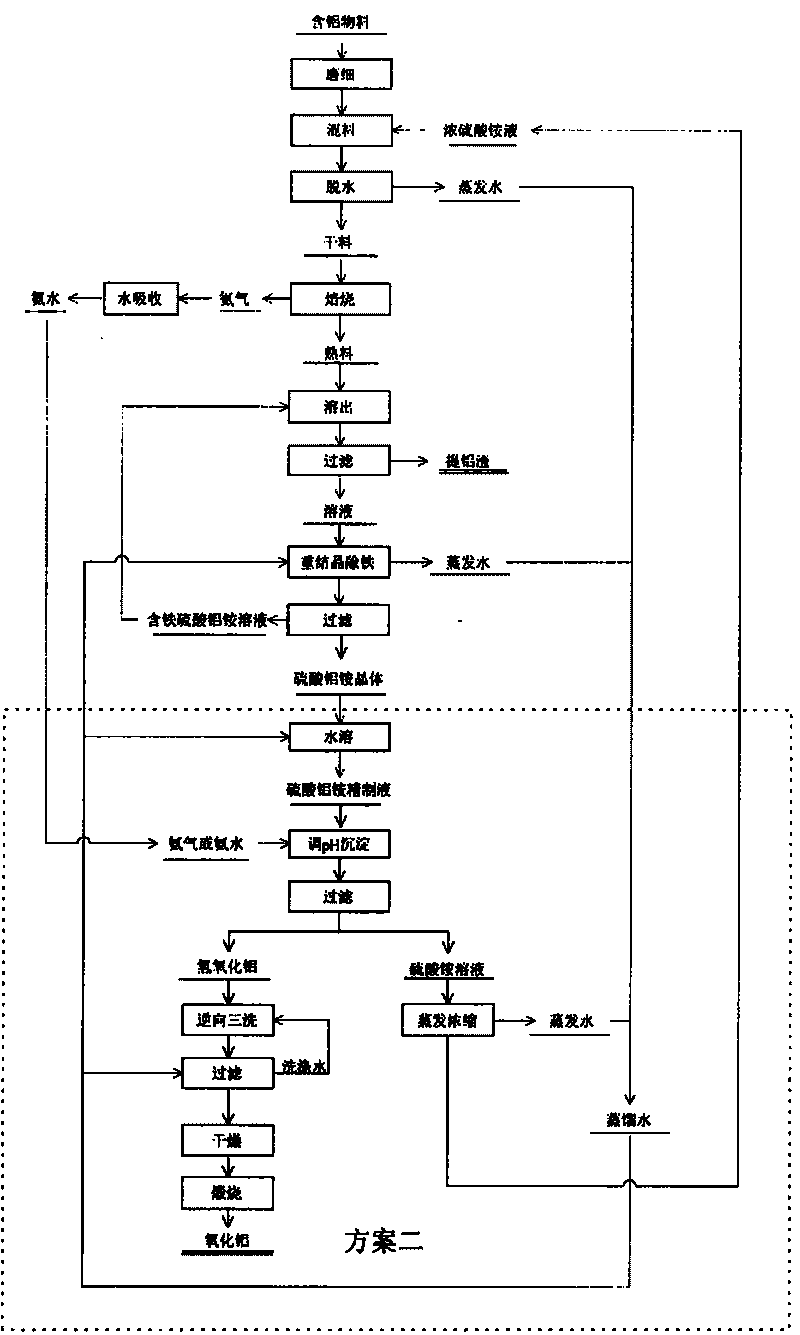
[0093] After the bauxite that has been crushed and ground to less than 80 μm is removed by magnetic separation, it is mixed with ammonium sulfate and water in a mass ratio of 1:5:7.2 (the molar ratio of alumina and ammonium sulfate is 1:6), and the ℃ roasting reaction for 6h, the gas released during the reaction is absorbed by water. The roasted product is cooled, dissolved in water, and then separated into solid and liquid. The filtrate is aluminum ammonium sulfate solution, and the filter residue is aluminum extraction slag.
[0094] After the ammonium aluminum sulfate solution is purified by recrystallization to remove impurities such as iron, pure ammonium aluminum sulfate crystals are prepared. Dissolve pure aluminum ammonium sulfate crystals with water, add ammonia water to control the [Al 3+ ] The initial concentration is 0.15mol L ...
Embodiment 3
[0097] The main composition of the high-iron and low-grade bauxite used is: Al 2 o 3 29.4%, SiO 2 8.9%, Fe 2 o 3 43.5%, TiO 2 1.6%, MnO 1.1%, K 2 O 0.78%.
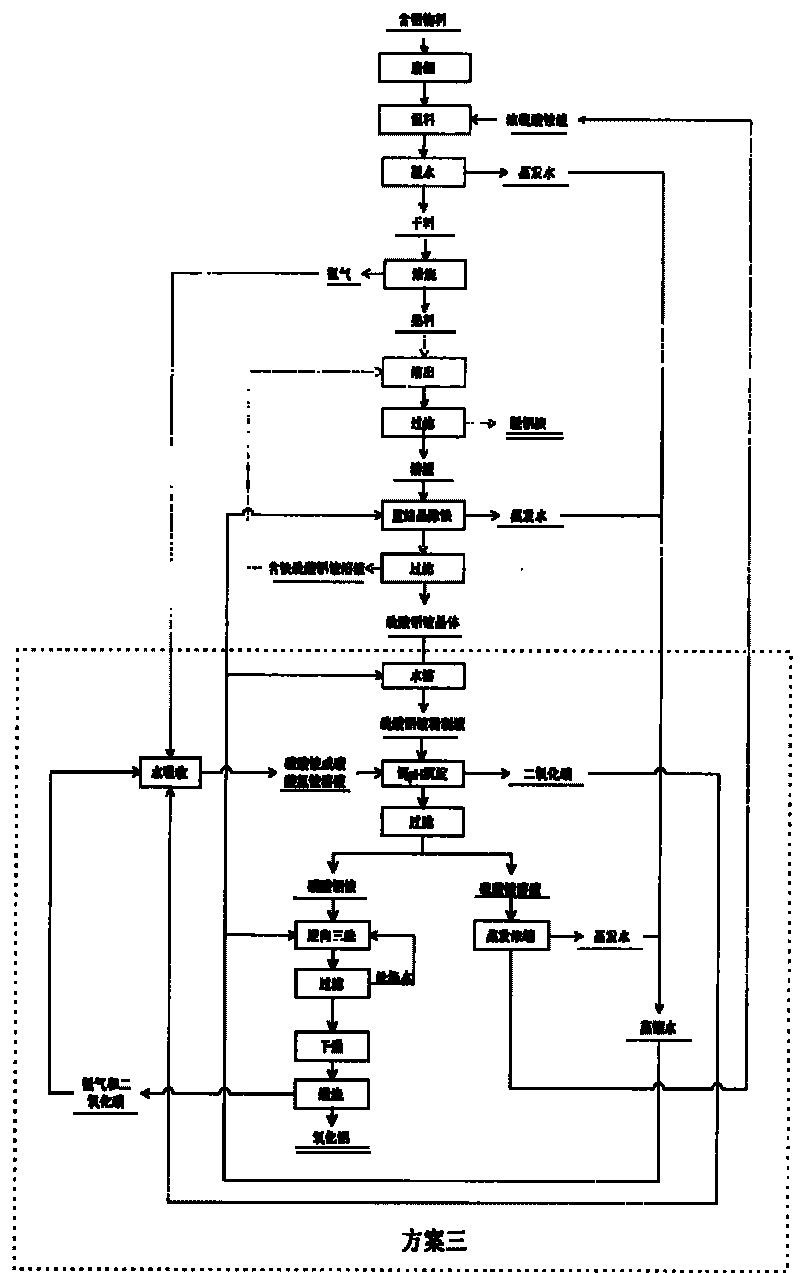
[0098] Mix the crushed and ground high-iron bauxite with ammonium sulfate and water at a mass ratio of 1:3:4.3 (the molar ratio of alumina and ammonium sulfate is 1:8), and roast at 500°C for 4 hours. The gas released during the reaction was absorbed with water. The roasted product is cooled, dissolved in water, and then separated into solid and liquid. The filtrate is aluminum ammonium sulfate solution, and the filter residue is aluminum extraction slag.
[0099] Feed ferrous oxide ions in the air into the ammonium aluminum sulfate solution, and use the yellow ammonium ironite method to remove iron. Add ammonia to the ammonium aluminum sulfate solution after iron removal to adjust the pH value to 4.5, and precipitate aluminum hydroxide. Aluminum hydroxide is heated to 1200°C and calcined for 3 hours to produ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com