Method for hydrogenation pyrolysis of prolific benzene and xylene by using pyrolysis gasoline
A technology for hydrocracking and pyrolysis of gasoline, which is applied in the petroleum industry, reforming naphtha, hydrocarbon cracking to produce hydrocarbons, etc., can solve problems such as low utilization value, achieve smooth orifices, avoid solvent extraction process, hydrogenation Moderate performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~4
[0021] Binder-free ZSM-5 zeolite (SiO 2 / Al 2 O 3 The molar ratio is 60). It is prepared with reference to Chinese patent CN1915820, and then ion exchanged with ammonium nitrate solution, and after the exchange, it is roasted and converted into hydrogen zeolite. The hydrogen type binderless ZSM-5 zeolite was impregnated with 0.04 parts by weight of Pt and 0.10 parts by weight of Sn, and calcined at 450° C. for 4 hours to obtain catalyst a.
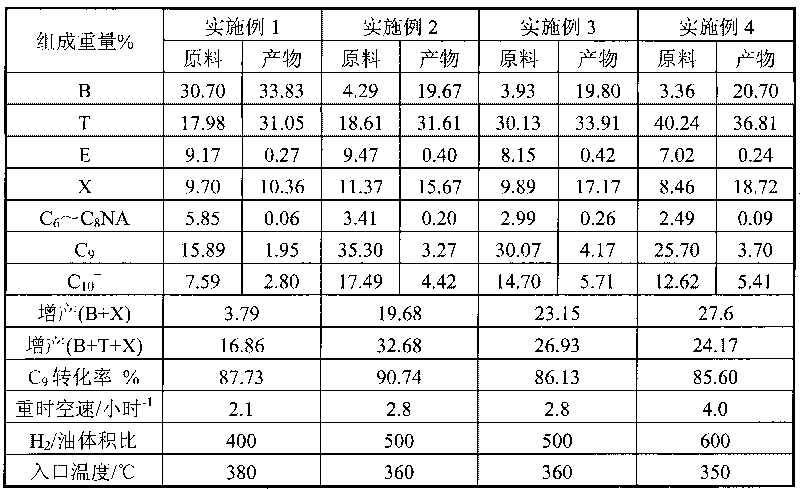
[0022] The reduction activated a catalyst was used for reaction evaluation. The catalyst was in a fixed bed reactor under a hydrogen pressure of 3.0MPa, H 2 / Oil volume ratio 400~600, reaction inlet temperature 350~380℃, weight hourly space velocity WHSV=2.0~4.0 hours -1 In the case of evaluation, the response results are listed in Table 1:
[0023] Table 1 Catalyst a reaction liquid phase product
[0024]
[0025] Where NA is non-aromatic hydrocarbon, C 10 + For carbon ten and above hydrocarbons, E is ethylbenzene.
Embodiment 1
[0026] The content of benzene and toluene in the raw material of Example 1 is relatively high. After the hydrocracking treatment, toluene has a large increase, while benzene and xylene have only a small increase; in Examples 2 to 4, as the benzene content in the raw material decreases, As the toluene content increases, both benzene and toluene increase after treatment, and the increase in toluene decreases. The toluene content of the raw material in Example 4 is the highest, and the toluene does not increase but decreases after treatment. This is because the conversion of aromatics in this reaction is limited by the reaction balance, and pyrolysis gasoline containing more benzene is used for treatment. The content of benzene in the raw material is very high, reaching 30-40%, so the increase of benzene after treatment Smaller. Adopt C 7 + The content of benzene in the pyrolysis gasoline raw material is small, so the increase of benzene after treatment is large. As the content of...
Embodiment 5~8
[0028] Respectively in the hydrogen type binderless ZSM-5 zeolite (SiO 2 / Al 2 O 3 The molar ratio is 90) on the load weight parts of 0.01 parts of Pt and 0.03 parts of Pb, 0.05 parts of Pt and 0.08 parts of Zn, 0.08 parts of Pt and 0.17 parts of Pd, 0.50 parts of Pd at 450° Calcined for 4 hours to obtain catalysts b, c, d and e.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com