Catalyst used for CO2 hydrogenation to directly produce gasoline and preparation method and application of the catalyst
A carbon dioxide and catalyst technology, which is applied in the field of catalysts and preparations for directly producing gasoline by hydrogenation of carbon dioxide, can solve the problems of complex phase structure, low Fischer-Tropsch activity and high selectivity of iron-based catalysts, and achieve high target product selectivity, High C5+ selectivity and high CO2 conversion effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
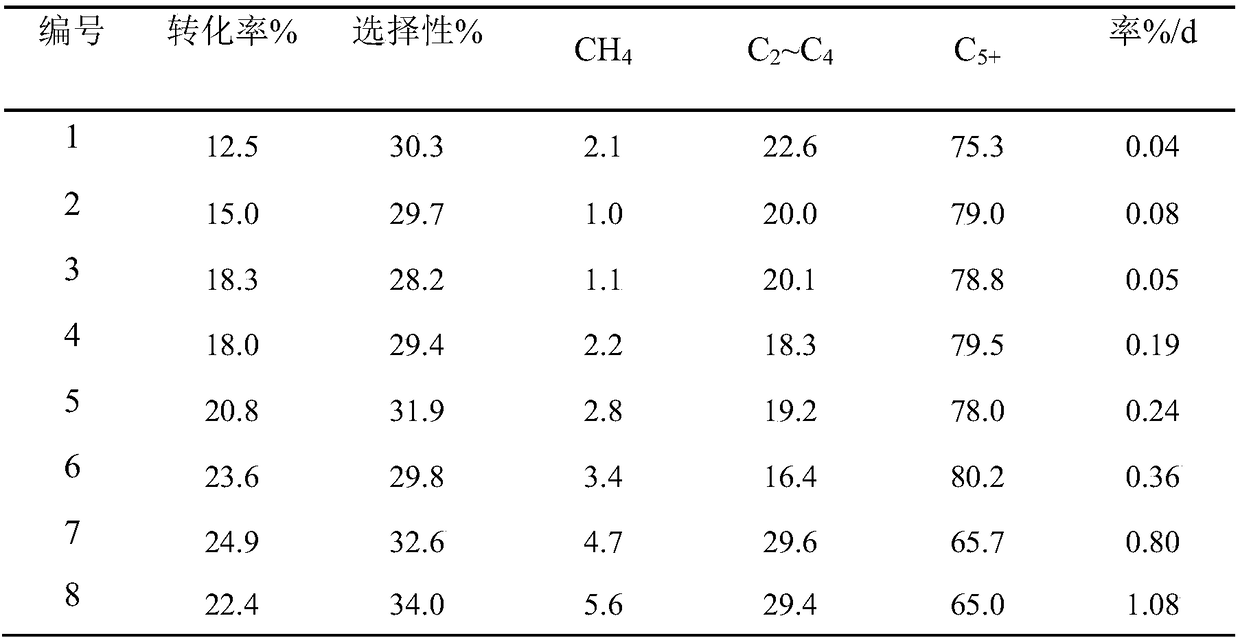
Examples
Embodiment 1
[0047] According to Zn / Zr=1:8 (molar ratio), 1.98g Zn(NO 3 ) 2 ·6H 2 O and 22.80g Zr(NO 3 ) 4 ·5H 2 O was added to 300mL deionized water to prepare a mixed metal salt solution with a concentration of 0.2mol / L, and 14.34g NaOH was added to 150mL ethanol to prepare a precipitant solution. The precipitant solution was added dropwise to the metal salt solution at a temperature of 10°C. After the precipitation reaction, the obtained product was aged at 40°C for 5 hours, and then the obtained precipitate was washed several times with deionized water. Then dry at 60°C for 24h, and then bake at 250°C for 12h to get ZnO-ZrO 2 metal oxide carrier. ZnO-ZrO 2 Grind into powder in an agate mortar for later use, according to the composition of the metal oxide catalyst In 2 o 3 Loading capacity is 10wt%, weighs 3.06g of In(NO 3 ) 3 .4.5H 2 O was dissolved in deionized water to prepare an impregnating solution, and impregnated the above 10g ZnO-ZrO 2 The powder is dried at 60°C f...
Embodiment 2
[0050] According to Zn / Zr=1:5 (molar ratio), 2.98g Zn(NO 3 ) 2 ·6H 2 O and 21.50g Zr(NO 3 ) 4 ·5H 2 O was added to 45mL deionized water and 15mL ethanol to prepare a mixed metal salt solution with a concentration of 1.0mol / L, and 23.04g (NH 4 ) 2 CO 3 Add to 150mL ethanol to prepare a precipitant solution. The precipitant solution was added dropwise to the metal salt solution at a temperature of 15° C. After the precipitation reaction, the obtained product was aged at 60° C. for 0.1 h, and then the obtained precipitate was washed several times with deionized water. Then dry at 60°C for 16h, and then bake at 250°C for 8h to get ZnO-ZrO 2 metal oxide carrier. ZnO-ZrO 2 Grind into powder in an agate mortar for later use, according to the composition of the metal oxide catalyst In 2 o 3 Loading capacity is 20wt%, weighs 6.88g of In(NO 3 ) 3 .4.5H 2 O was dissolved in deionized water to prepare an impregnating solution, and impregnated the above 10g ZnO-ZrO 2 powder...
Embodiment 3
[0053] According to Zn / Zr=1:4 (molar ratio), 3.60g Zn(NO 3 ) 2 ·6H 2 O and 20.80g Zr(NO 3 ) 4 ·5H 2 O was added to 112.5mL deionized water and 37.5mL ethanol to prepare a mixed metal salt solution with a concentration of 0.4mol / L, and 29.19g ammonia water (NH 3 The concentration is 28wt%) joins in the 150mL ethanol and prepares the precipitating agent solution. The precipitant solution was added dropwise to the metal salt solution at a temperature of 15° C. After the precipitation reaction, the obtained product was aged at 60° C. for 0.5 h, and then the obtained precipitate was washed several times with deionized water. Then dry at 70°C for 12h, and then bake at 250°C for 6h to get ZnO-ZrO 2 metal oxide carrier. ZnO-ZrO 2 Grind into powder in an agate mortar for later use, according to the composition of the metal oxide catalyst In 2 o 3 Loading capacity is 20wt%, weighs 6.88g of In(NO 3 ) 3 .4.5H 2 O was dissolved in deionized water to prepare an impregnating sol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com