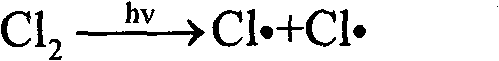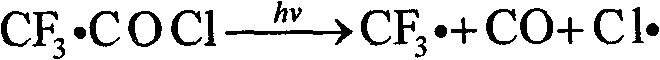Method for preparing trifluoroacetyl chloride
A technology of trifluoroacetyl chloride and chlorine gas, applied in the preparation of acyl halide, organic chemistry and other directions, can solve the problems of poor selectivity, unsafe, difficult separation, etc., and achieve the effect of avoiding corrosion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Add 1L of HCFC-123 into a 1L special three-neck glass flask, and place a set of shading plates below the gas-liquid phase interface. The shading plates are similar to the louver structure, allowing the gas to pass through but preventing the light under the liquid from penetrating into the gas phase space. The middle interface on the top of the flask is the mercury lamp placement port, and the 500w mercury lamp, quartz cold hydrazine, and quartz sleeve are placed in sequence from the inside to the outside, and the sleeve and the interface are sealed. Oxygen and chlorine are fed continuously under magnetic stirring, the flow rate is 75ml / min and 15ml / min respectively, after 15 minutes, the mercury lamp is turned on, and the photochemical oxidation reaction is carried out through the radiation of the mercury lamp, and the reaction is carried out at normal temperature and pressure. The reactant is continuously discharged from the gas phase outlet on the upper part of the rea...
Embodiment 2
[0032] The difference of embodiment 2 reaction and experiment 1 is that no shading plate is added below the gas-liquid phase interface of embodiment 2, and other conditions are the same as experiment 1, and TFAC accounts for 90% except raw material, oxygen, chlorine and hydrogen chloride gas in the gas phase product, altogether 0.94 mol TFAC was produced. The content of HCFC-123 in the bottom solution is 93.14%, and the by-product CFC-113a is 6.81%. Glass corrosion was found at the gas-liquid phase interface of the reactor, the gas phase part, and the condensation return pipe.
Embodiment 3
[0034] The difference between reaction and experiment 1 in embodiment 3 is that the flow of chlorine gas in embodiment 3 is adjusted to 0 after the mercury lamp is turned on, and other conditions are the same as in experiment 1. In the gas phase product, TFAC accounts for 99% except raw materials, oxygen, chlorine and hydrogen chloride gas. %, a total of 0.64mol TFAC was produced. The content of HCFC-123 in the bottom liquid is 98.59%, and the by-product CFC-113a is 1.27%. No corrosion was found in the glass of the reactor, condenser, etc.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com