Tablet containing faropenem sodium
A technology of faropenem sodium and tablets, which is applied in the field of pharmaceutical preparations and can solve problems such as poor stability and difficulty in swallowing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
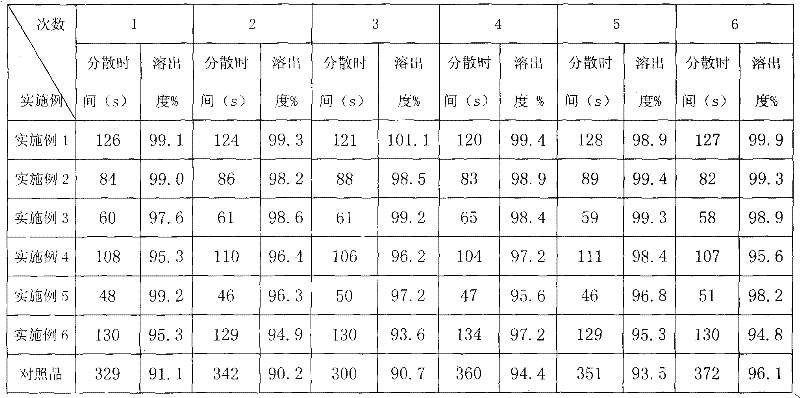
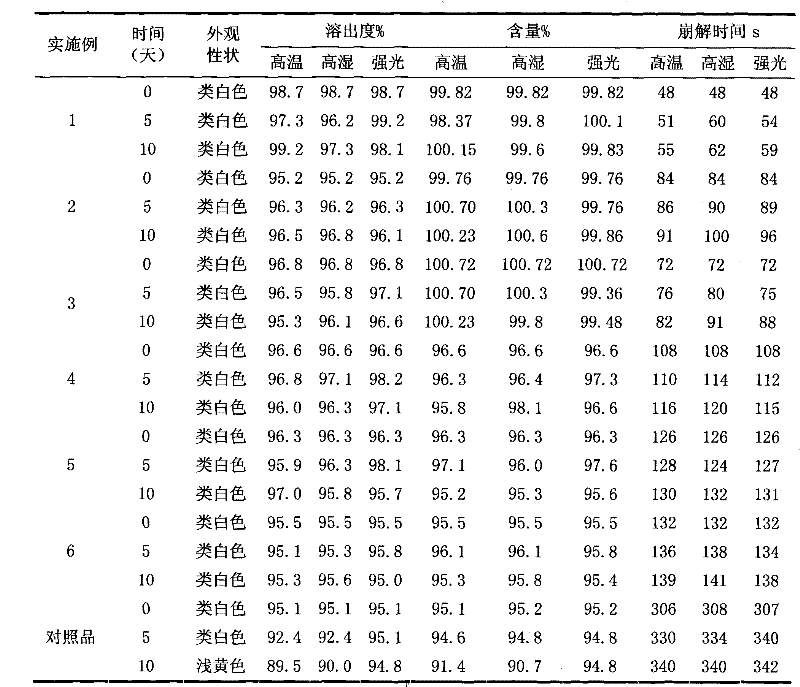
[0039] Embodiment 1 Faropenem sodium tablet
[0040] Faropenem sodium (main ingredient) 123.5g
[0041] Microcrystalline cellulose (filler) 44g
[0042] Micronized silica gel (filler) 12g
[0043] Cross-linked polyvinylpyrrolidone (disintegrant) 64g
[0044] Low-substituted hydroxypropyl cellulose (disintegrant) 20g
[0045] Aspartame 4g
[0046] 5% PVPk30 95% ethanol solution appropriate amount
[0047] Magnesium stearate (lubricant) 0.8%
[0048] A total of 1000 tablets were produced, each tablet weighed 0.28g, and the main drug was 0.10g calculated as faropenem.
[0049] Preparation process: crush the raw material through a 100-mesh sieve; crush the rest of the excipients through a 80-mesh sieve; accurately weigh the raw and excipients in the prescribed amount, mix them evenly, granulate with 5% PVPk3095% ethanol solution, granulate with a 18-mesh sieve, ℃ dry. Add the prescribed amount of magnesium stearate, mix evenly, pass through a 18-mesh sieve, measure the con...
Embodiment 2
[0050] Example 2 Faropenem Sodium Tablets
[0051] Faropenem sodium (main ingredient) 123.5g
[0052] Microcrystalline cellulose (filler) 72g
[0053] Micronized silica gel (filler) 12g
[0054] Cross-linked polyvinylpyrrolidone (disintegrant) 36g
[0055] Low-substituted hydroxypropyl cellulose (disintegrant) 20g
[0056] Aspartame 4g
[0057]5% PVPk30 95% alcohol solution appropriate amount
[0058] Magnesium stearate (lubricant) 0.8%
[0059] A total of 1000 tablets were produced, each tablet weighed 0.28g, and the main drug was 0.10g calculated as faropenem.
[0060] Preparation process: crush the raw material through a 100-mesh sieve; crush the rest of the excipients through a 80-mesh sieve; accurately weigh the raw and excipients in the prescribed amount, mix them evenly, granulate with 5% PVPk3095% ethanol solution, granulate with a 18-mesh sieve, ℃ dry. Add the prescribed amount of magnesium stearate, mix evenly, pass through a 18-mesh sieve, measure the conten...
Embodiment 3
[0061] Example 3 Faropenem Sodium Tablets
[0062] Faropenem sodium (main ingredient) 123.5g
[0063] Microcrystalline cellulose (filler) 60g
[0064] Micronized silica gel (filler) 10g
[0065] Cross-linked polyvinylpyrrolidone (disintegrant) 60g
[0066] Low-substituted hydroxypropyl cellulose (disintegrant) 10g
[0067] Aspartame 4g
[0068] 5% PVPk30 95% ethanol solution appropriate amount
[0069] Magnesium stearate (lubricant) 0.8%
[0070] A total of 1000 tablets were produced, each tablet weighed 0.28g, and the main drug was 0.10g calculated as faropenem.
[0071] Preparation process: crush the raw material through a 100-mesh sieve; crush the rest of the excipients through a 80-mesh sieve; accurately weigh the raw and excipients in the prescribed amount, mix them evenly, granulate with 5% PVPk3095% ethanol solution, granulate with a 18-mesh sieve, ℃ dry. Add the prescribed amount of magnesium stearate, mix evenly, pass through a 18-mesh sieve, measure the conte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com