Synthetic technology of p-methoxystyrene
A technology of methoxystyrene and a synthesis process, applied in the chemical field, can solve problems such as long reaction period, unfavorable industrial continuous production, etc., and achieve the effects of low production cost, little environmental pollution, and reduced production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The concrete processing step of the inventive method is described as follows now:
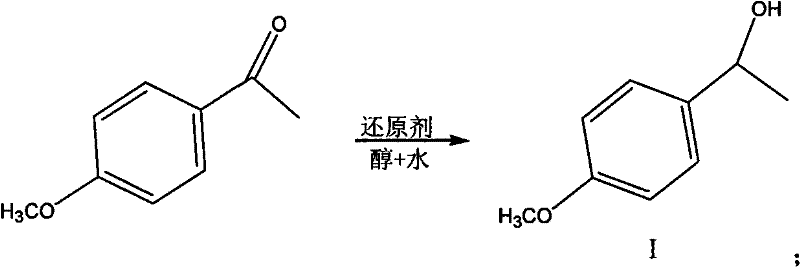
[0025] a. Add 500g of p-methoxyacetophenone, 150ml of ethanol and 150ml of water to a three-necked flask equipped with mechanical stirring and a serpentine condenser, raise the temperature to 80°C, add 52g of potassium borohydride in batches within 30min, and react 4 Hour. Add 300ml of water to wash off the excess potassium borohydride, let stand to separate the liquid to remove the water layer, add anhydrous magnesium sulfate to remove the residual water, and use a rotary evaporator to evaporate the ethanol under reduced pressure to obtain compound I, which is p-methoxy-α-formazol 490.5g of benzyl alcohol. The yield is 96.8%, and the content detected by GC is 99.5%.
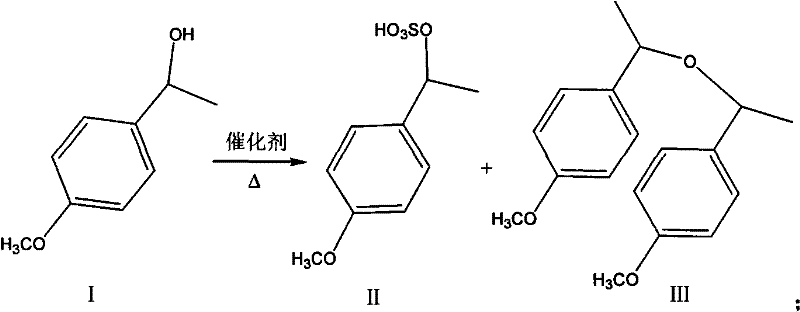
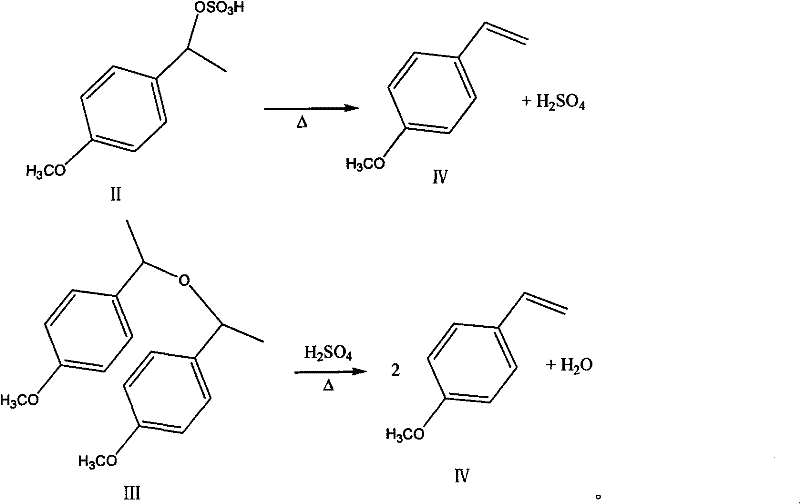
[0026] b. Add 12.5 g of p-methoxy-α-methylbenzyl alcohol and 0.52 g of potassium bisulfate to a three-necked flask equipped with mechanical stirring, raise the temperature to 60° C., and stir for 2.5 hours. Unreacted po...
Embodiment 2
[0029] This example is basically the same as Example 1, except that in step a, the temperature is 65° C., and the reaction time is 6 hours. 493.8 g of p-methoxy-α-methylbenzyl alcohol was obtained. The yield is 97.3%, and the content detected by GC is 99.1%.
Embodiment 3
[0031] This embodiment is basically the same as Embodiment 1, except that in step a, the solvent alcohol used is propanol, 150ml of propanol and water. 490 g of p-methoxy-α-methylbenzyl alcohol was obtained. The content detected by GC was 99.0%, and the yield was 95.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com