Monoclonal antibody (hybrid tumor) for neutralizing CYR61 and applications thereof
A monoclonal antibody and hybridoma technology, applied in the field of hybridoma, can solve the problems of inability to use in vivo, imperfect long-term transfection technology of SiRNA, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
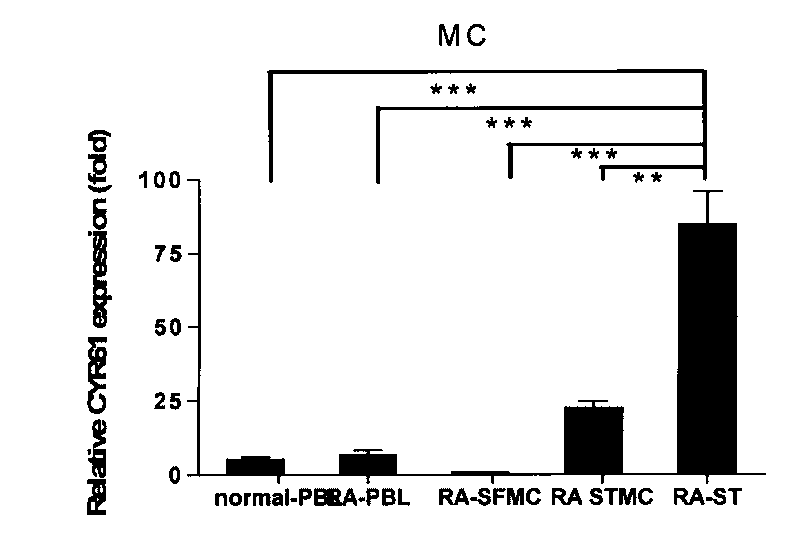
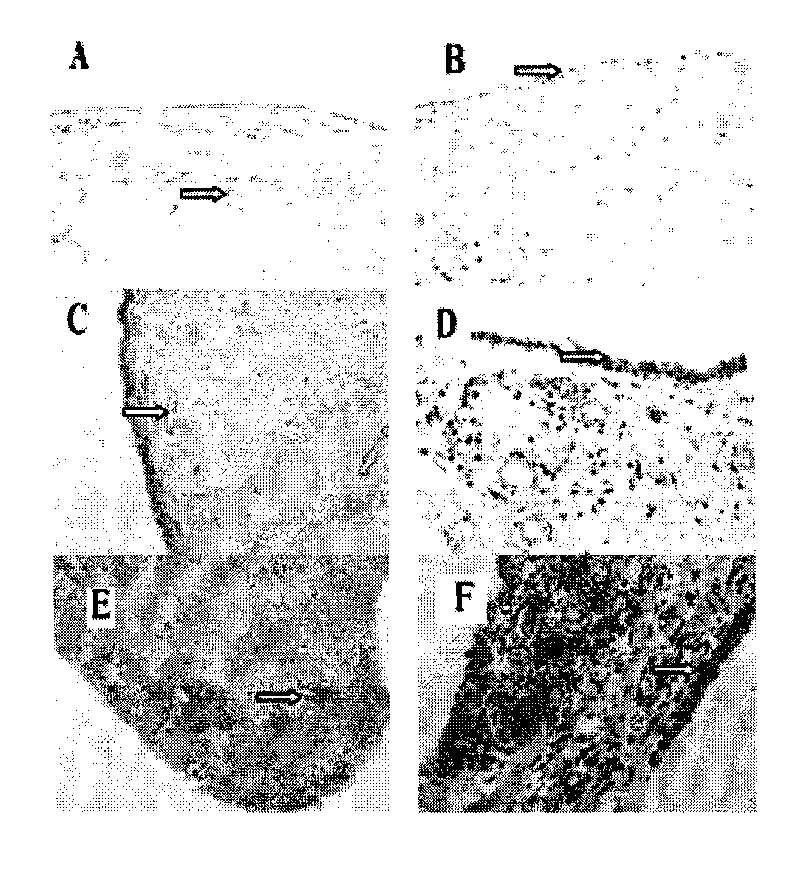
[0046] Example 1: Expression Pattern of CYR61 in Synovial Tissue of Rheumatoid Arthritis
[0047] 1. Materials and methods
[0048] 1 Experimental materials
[0049] 1.1 Clinical samples:
[0050] All samples in the study were from RA patients and OA patients undergoing knee replacement or synovectomy in clinical orthopedics department, and the diagnosis of all cases met the international diagnostic criteria. Two normal controls were from trauma patients. The patient's synovial tissue was used for cell culture in vitro, part of the tissue was fixed with 4% paraformaldehyde and reserved for immunohistochemistry, serum and synovial fluid (SF) were centrifuged at high speed to obtain supernatant, and stored at -80°C. All clinical samples used in this study were informed to the patients.
[0051] 2 Experimental methods
[0052] 2.1 Cell preparation
[0053] 2.1.1 Preparation of peripheral blood mononuclear cells:
[0054] Mononuclear cells were separated from heparin-antico...
Embodiment 2
[0080] Example 2: CYR61 in rheumatoid arthritis synovial fluid promotes the proliferation of FLS
[0081] 1. Materials and methods
[0082] 1.1 Synovial fluid stimulation experiment: Mix 3-5 parts of synovial fluid and add to FLS in logarithmic growth phase to continue culturing for 24 hours, then detect CYR61 mRNA and protein expression levels, or FLS proliferation (see below for details).
[0083] 1.2 Cell proliferation and antibody blocking experiments: take FLS in the logarithmic growth phase, digest with 0.25% trypsin and adjust the cell concentration to 1×10 4 cells / ml, inoculate in 96-well cell culture plate, add different concentrations of SF (the ratio of SF to culture medium is 1:32, 1:16, 1:8, 1:4, 1:2) or cytokines (IL-10, IL12, IFNγ, TNFa and IL-17), or pure CYR61 protein was co-cultured with FLS. 1 μCi of 3H (per well) was added 16 hours before the end of the culture, the cells were collected, and the proliferation was detected by a β liquid scintillation instr...
Embodiment 3
[0097] Example 3: Study on the mechanism of CYR61 promoting the proliferation of rheumatoid arthritis FLS
[0098] 1. Materials and methods
[0099] 1. Clinical samples, CYR61 gene and protein expression detection are the same as those described in the materials and methods of "Example 1: CYR61 expression pattern in rheumatoid arthritis synovial tissue".
[0100] 2. FLS culture and proliferation stimulation experiments are the same as those described in the materials and methods of "Example 2: CYR61 promotes the proliferation of FLS in rheumatoid arthritis synovial fluid".
[0101] 3. SiRNA interference experiment:
[0102] 3.1: SiRNA sequence and synthesis: Synthesize 3 siRNAs according to the siRNA design principle, the sequence is as follows:
[0103] S1: 5'-CCA GAA AUG UAU UGU UCA ATT-3-
[0104] 5’-UUG AAC AAU ACA UUU CUG GCC-3-
[0105] S2: 5’-UCA AGG UAU CAA UGU UUA ATT-3-
[0106] 5’-UUG CUC GCA ACA AAU CUG CTC-3-
[0107] S3: 5'-CAA CGA GGA CUG CAG CAA ATT-3-
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com