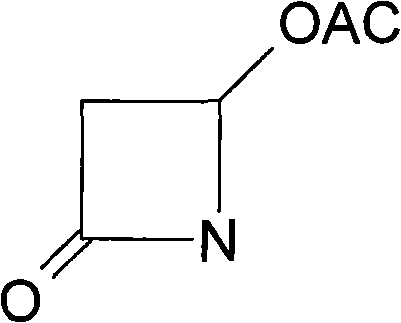
Method for preparing 4-acetoxy-2-azetidinone
A technology of azetidinone and acetoxy, applied in the field of producing 4-acetoxy-2-azetidinone, can solve the problems of many side reactions, trivial and troublesome post-processing, low product quality, etc. Reasonable and effective post-processing, easy industrial production, simple process and equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0021] 1. In a 20L reaction kettle, under the protection of nitrogen, add 3L of vinyl acetate, cool to -15°C, add 850g of chlorosulfonyl isocyanate within 10 minutes, stir during the addition and keep the temperature at about -10°C, then Raise the temperature to 10°C and react for 40 minutes to obtain a dark red mixture, then lower the temperature to below -20°C.
[0022] 2. Add 700g of sodium bisulfite under stirring, control the temperature at about -10°C, add the aqueous solution of 1.3kg of sodium bicarbonate and 1.4kg of sodium bisulfite prepared with 4L of water in batches, and stir and react at about -10°C for 60min .
[0023] 3. Filter the reaction mixture (suction filtration or centrifugation), wash the filter cake with 2×400L vinyl acetate, discard the filter cake, take the filtrate and let it stand for stratification, separate the upper organic layer, and then wash the water layer with vinyl acetate 3 × 1.0L extraction, combined organic layers, with anhydrous MgSO ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com