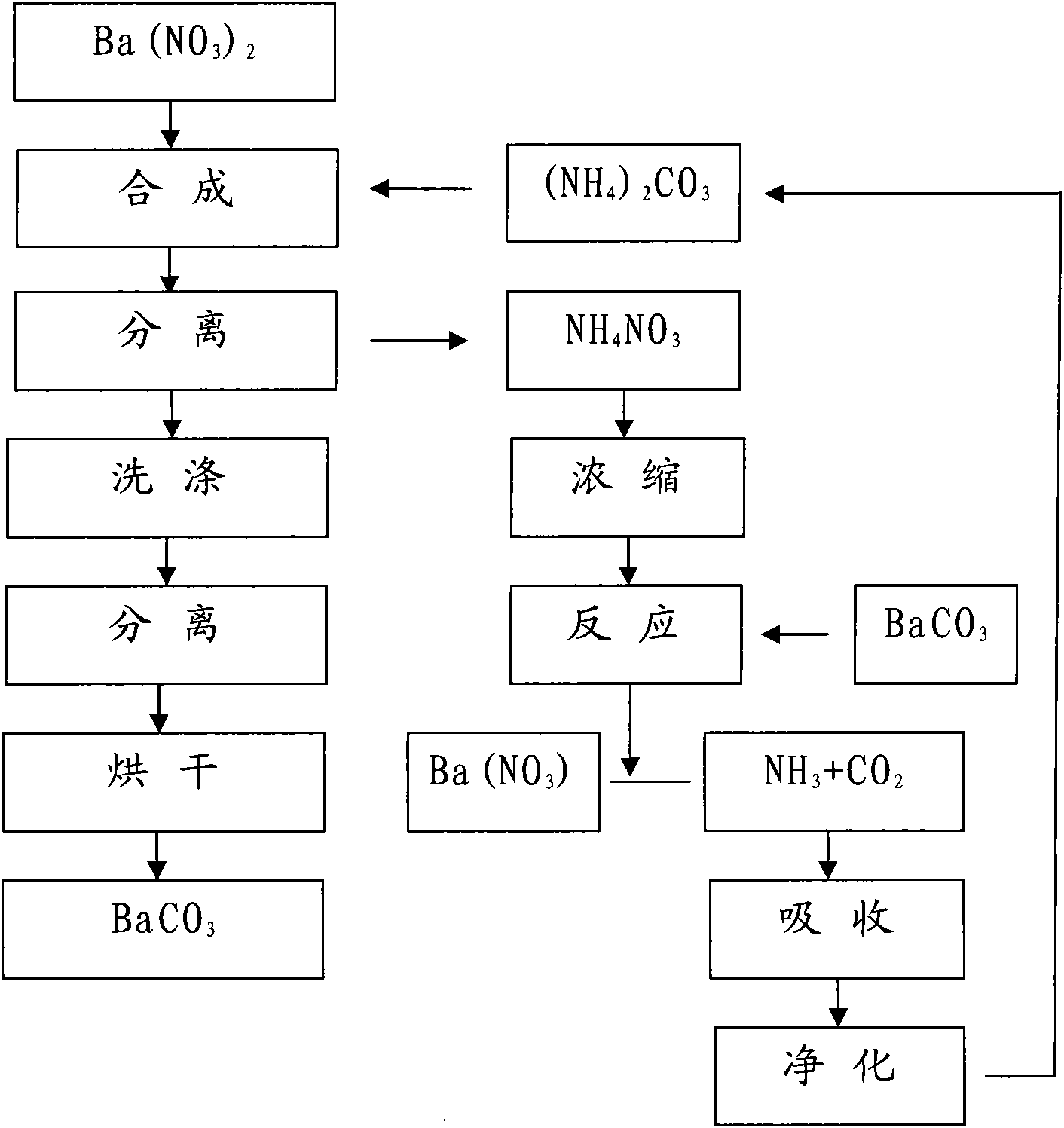
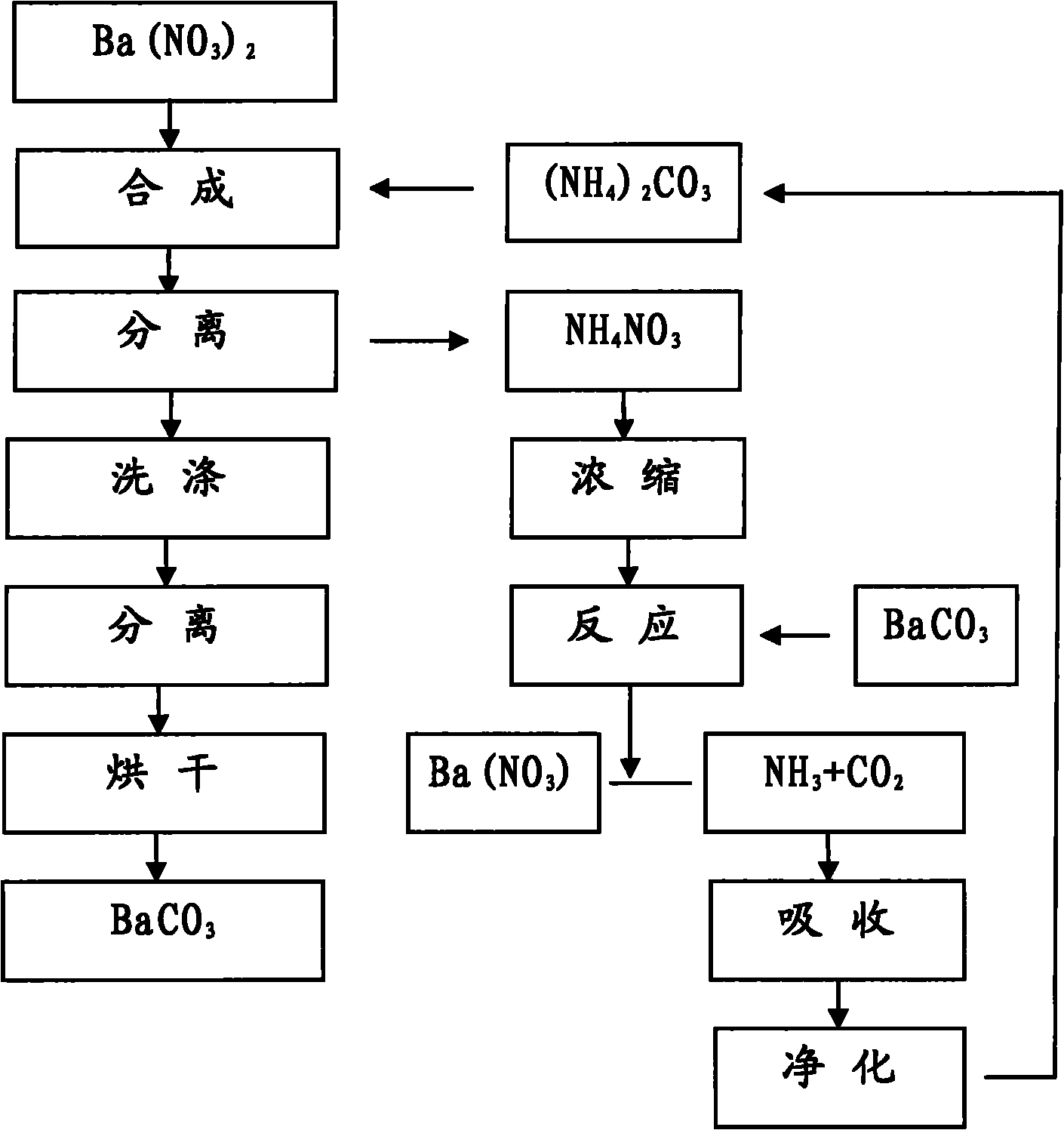
Method for producing BaCO3 through cyclically recovering and reusing (NH4)2CO3
A technology of recycling and absorbing liquid, applied in the direction of ammonium carbonate/acid carbonate, calcium carbonate/strontium/barium, etc., can solve the problem of high energy consumption, difficulty in washing chloride ions, and difficulty in handling high-impurity mother liquor, etc. problem, to achieve the effect of simple washing and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Will [Ba 2+ ] 3000ml of 0.85mol / L barium nitrate solution is placed in a 5000ml beaker, heated to boil, stop heating, add 3.0mol / L (NH 4 ) 2 CO 3 900ml, the adding speed is controlled at about 1.5L / hour.
[0018] Suction filter and separate the above synthetic materials, rinse with a small amount of hot deionized water, combine the filtrate into the recovery system, heat wash the filter cake at 100°C for 1 hour at a material-to-water ratio of 1:5, separate by suction filtration, and place it in an oven at 180°C for drying Dry for 16 hours to obtain BaCO 3 sample 1 # .
Embodiment 2
[0020] Will [Ba 2+ ] 3000ml of 0.83mol / L barium nitrate solution is placed in a 5000ml beaker, heated to boil, stop heating, add 2.15mol / L (NH 4 ) 2 CO 3 1220ml, control the adding speed at about 2.0L / hour.
[0021] The above-mentioned synthetic materials are separated by suction filtration, rinsed with a small amount of hot deionized water, and the combined filtrate enters the recovery system. The filter cake is heated at 100°C for 1 hour at a material-to-water ratio of 1:5, then separated by suction filtration, and dried in an oven at 180°C. Dry for 16 hours to obtain BaCO 3 sample 2 # .
Embodiment 3
[0023] Will [Ba 2+ ] 3000ml of 0.40mol / L barium nitrate solution is placed in a 5000ml beaker, heated to boil, stop heating, add 2.0mol / L (NH 4 ) 2 CO 3 630ml, control the adding speed at about 2.0L / hour.
[0024] The above-mentioned synthetic materials are separated by suction filtration, rinsed with a small amount of hot deionized water, and the combined filtrate enters the recovery system. The filter cake is heated at 100°C for 1 hour at a material-to-water ratio of 1:5, then separated by suction filtration, and dried in an oven at 180°C. Dry for 16 hours to obtain BaCO 3 sample 3 # .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com