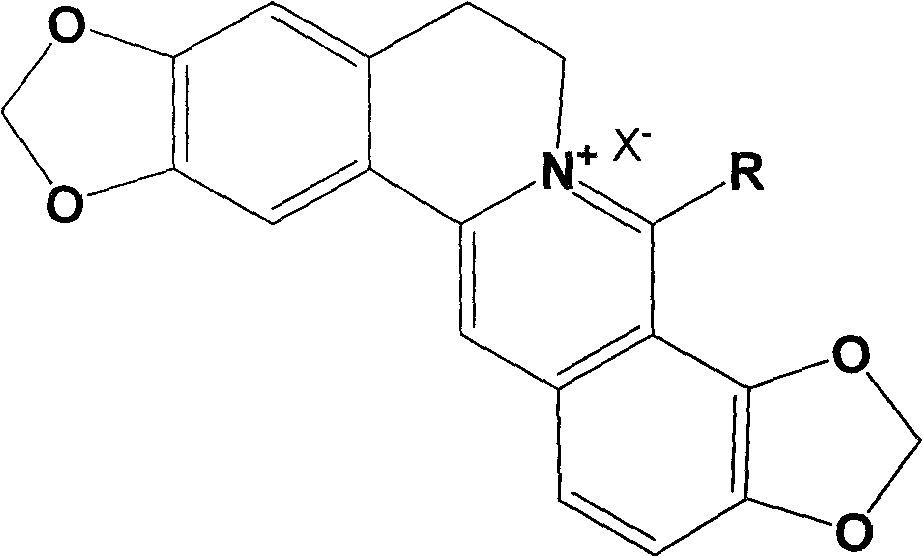
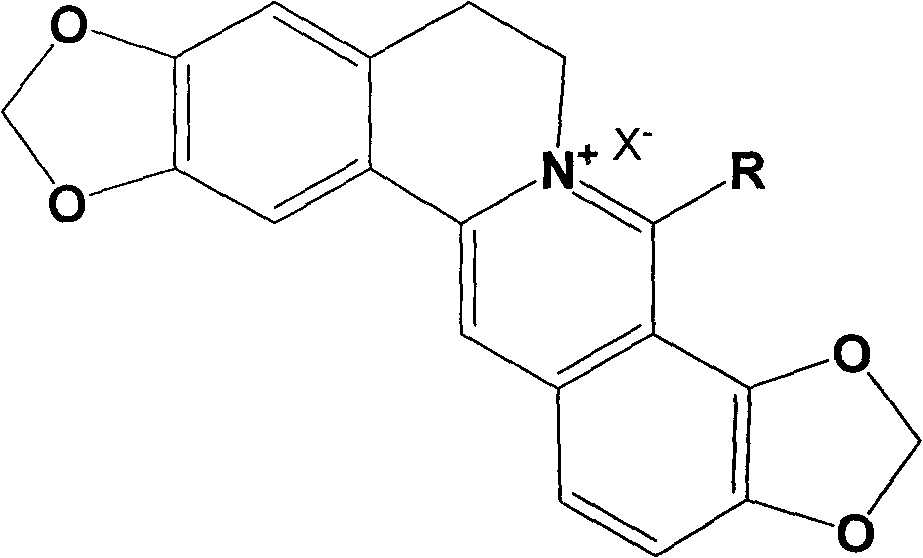
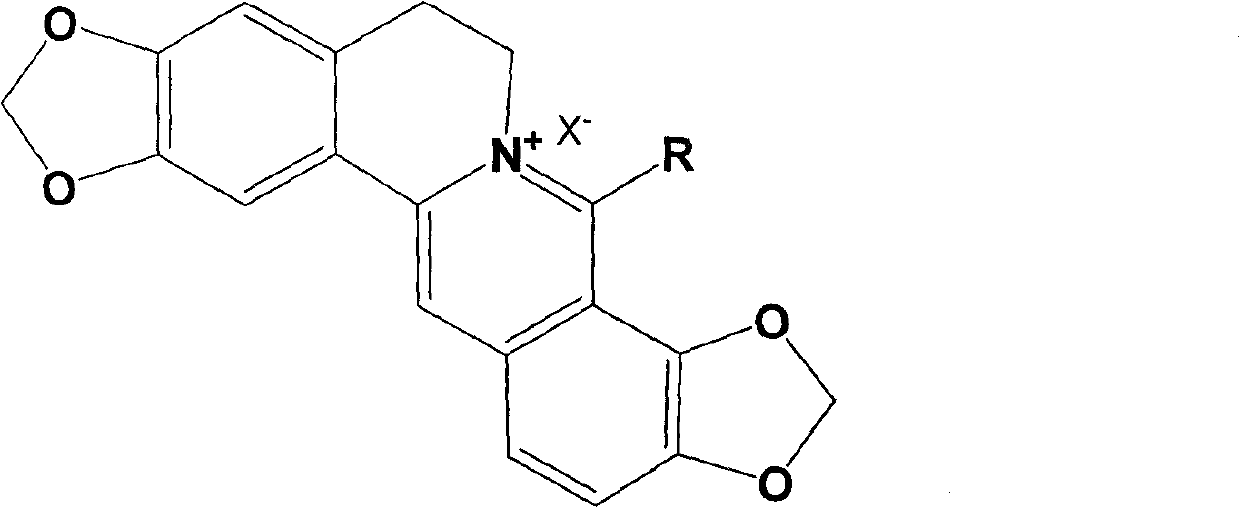
Long-chain alkyl coptisine halate derivative, synthesis method and application
The technology of a long-chain alkyl group and a synthesis method is applied in the field of derivatives of long-chain alkyl coptis base salts, and can solve problems such as no literature reports, and achieve a high blood lipid-lowering effect, which is beneficial to human body absorption and high in yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: 8-methyl coptisine iodate.
[0032]
[0033] It is prepared as follows:
[0034] ①. Dry all the reaction glass instruments, weigh 0.1mol of dried magnesium chips and place in a 250mL three-necked flask, use 40mL of anhydrous tetrahydrofuran as the reaction solvent, add 0.1mol of n-butane iodide under nitrogen protection, and prepare the corresponding Grignard reagent.
[0035] ②. Weigh 0.1 mol of dry berberine hydrochloride and put it in a 500mL three-neck flask, add 40mL of anhydrous tetrahydrofuran to make a suspension of berberine hydrochloride, and put it in an ice bath under nitrogen protection to -10°C.
[0036] ③. Slowly add the prepared Grignard reagent into the coptisine hydrochloride suspension under nitrogen protection and ice bath, while stirring. After the addition, remove the ice bath, return to room temperature, and heat to reflux for 2 hours to complete the reaction.
[0037] ④, centrifuge the reaction solution, take the supernatant, then...
Embodiment 2
[0040] Example 2: 8-butyl coptisine hydrochloride.
[0041]
[0042] It is prepared as follows:
[0043] ①. Dry all the reaction glass instruments, weigh 0.15mol of dried magnesium chips and place in a 250mL three-neck flask, use 40mL of anhydrous tetrahydrofuran as the reaction solvent, add 0.1mol of n-chlorobutane under nitrogen protection, and prepare the corresponding Grignard reagent.
[0044] ②. Weigh 0.01mol of dry berberine hydrochloride and place it in a 500mL three-neck flask, add 500mL of anhydrous tetrahydrofuran to make a suspension of berberine hydrochloride, and then place it in an ice bath under nitrogen protection to -10°C.
[0045] ③. Slowly add the prepared Grignard reagent into the coptisine hydrochloride suspension under nitrogen protection and ice bath, while stirring. After the addition, remove the ice bath, return to room temperature, and heat to reflux for 2 hours to complete the reaction.
[0046] ④, centrifuge the reaction solution, take the sup...
Embodiment 3
[0049] Example 3: 8-octyl coptisine bromate.
[0050]
[0051] It is prepared as follows:
[0052] ①. Dry all the reaction glass instruments, weigh 0.13mol of dried magnesium chips and place in a 250mL three-necked flask, use 40mL of anhydrous tetrahydrofuran as the reaction solvent, add 0.1mol of brominated n-octane under nitrogen protection, and prepare the corresponding Grignard reagent.
[0053] ②. Weigh 0.05mol of dry berberine hydrochloride and place it in a 500mL three-necked flask, add 1000mL of anhydrous tetrahydrofuran to form a berberine hydrochloride suspension, and then place it in an ice bath under nitrogen protection to -10°C.
[0054] ③. Slowly add the prepared Grignard reagent to the Coptis base suspension under nitrogen protection and ice bath, while stirring, remove the ice bath after the addition, return to room temperature, and heat to reflux for 2 hours to complete the reaction.
[0055] ④, centrifuge the reaction solution, take the supernatant, then...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com