Chromium hydroxide, method for producing the same, trivalent chromium-containing solution using the same, and chromium plating method
A technology for chromium hydroxide, manufacturing methods, applications in chromium oxide/hydrate, nanotechnology for materials and surface science, transportation and packaging, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~6
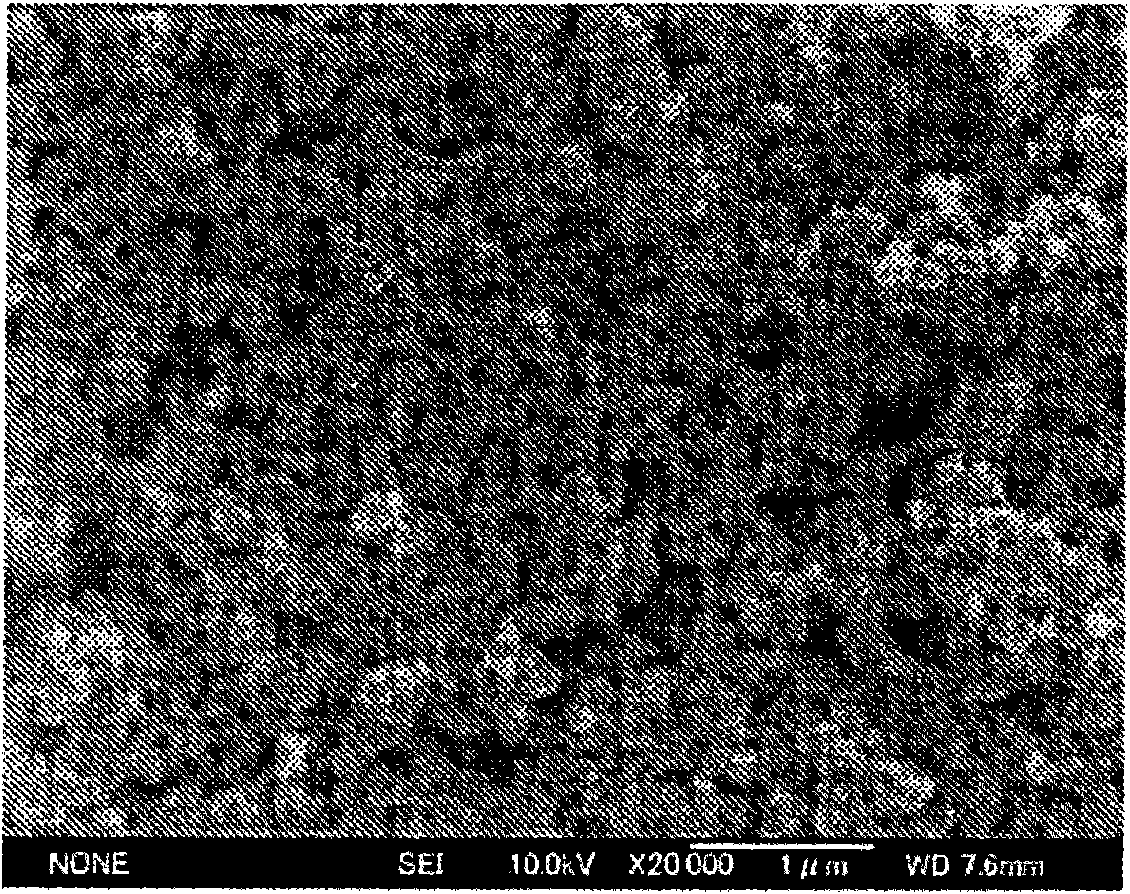
[0080] 140 g of 10% sodium hydroxide aqueous solution and 52 g of 35% chromium chloride aqueous solution (manufactured by Nippon Chemical Industry Co., Ltd.) were added to the container and diluted with 208 g of water to obtain a 7% chromium chloride aqueous solution for preparation. Next, the aqueous sodium hydroxide solution and the aqueous chromium chloride solution were adjusted to the temperatures shown in Table 1. While stirring the sodium hydroxide aqueous solution at the speed shown in Table 1, the chromium chloride aqueous solution was added thereto at the speed shown in Table 1. Addition was performed until the pH of the reaction system reached the value shown in the same table. The generated precipitate was filtered and washed with water at 30° C. until the conductivity of the filtrate reached 1 mS / cm to obtain about 12 g of chromium hydroxide. This chromium hydroxide was suspended in pure water to obtain a slurry with a concentration of 8%. The solubility and deg...
Embodiment 7~9 and comparative example 14~17
[0093] Except having set the stirring rate at the time of adding the chromium chloride aqueous solution to the conditions shown in Table 4, the same operation as Examples 1-6 was performed, and the slurry containing chromium hydroxide and chromium hydroxide was obtained. The solubility, average particle diameter, and degree of aggregation of the obtained chromium hydroxide were measured in the same manner as in Examples 1 to 6. However, the solubility was measured immediately after generation of chromium hydroxide, and was measured after storage for 7 days. The results are shown in Table 4. In these comparative examples, since the stirring speed was lower than that of the examples, the amount of trivalent chromium was locally excessive relative to the amount of the alkali, and the obtained chromium hydroxide had poor solubility in acidic solutions.
[0094] [Table 4]
[0095]
Embodiment 10
[0097] Except having used 195 g of 10% potassium hydroxide aqueous solution instead of sodium hydroxide aqueous solution, the same operation as Example 3 was performed, and the slurry containing chromium hydroxide and chromium hydroxide was obtained. The solubility, average particle diameter, and degree of aggregation of the obtained chromium hydroxide were measured in the same manner as in Examples 1 to 6. The results are shown in Table 5.
[0098] [table 5]
[0099]
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com