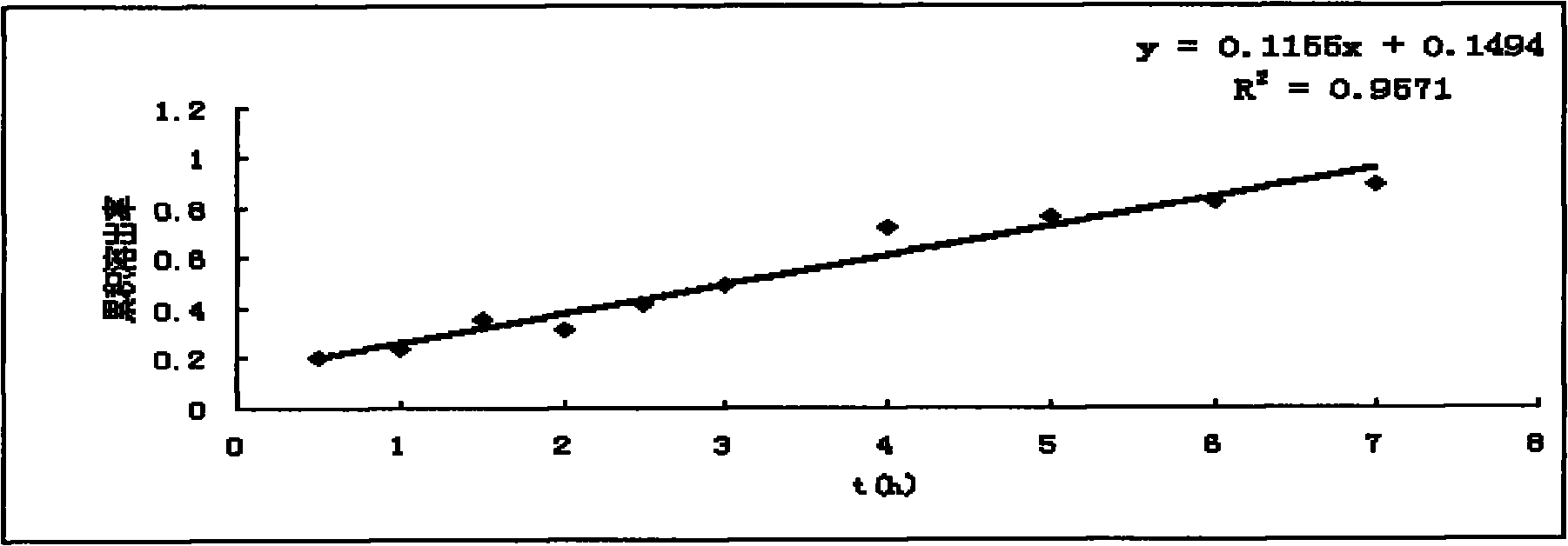
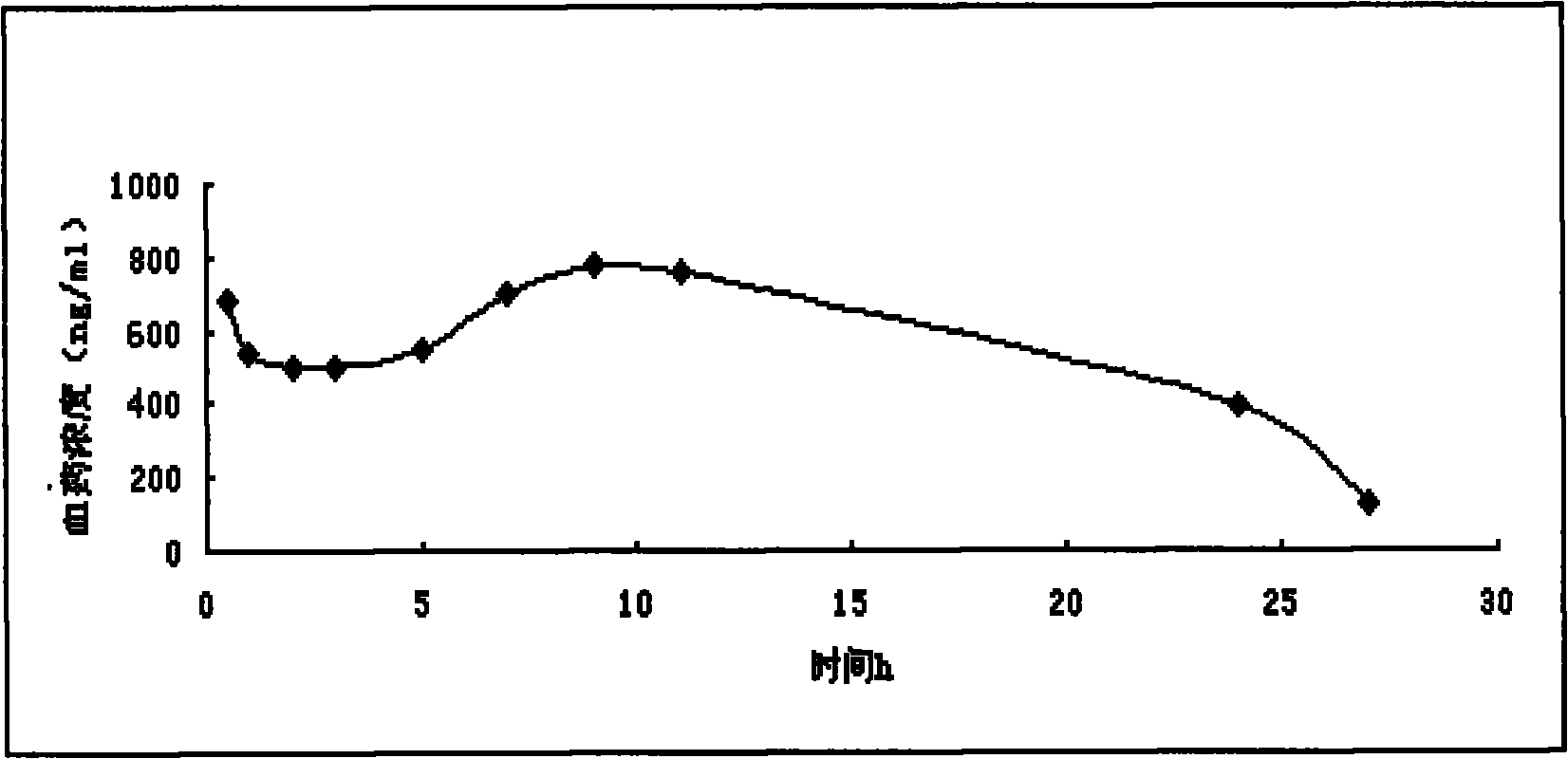
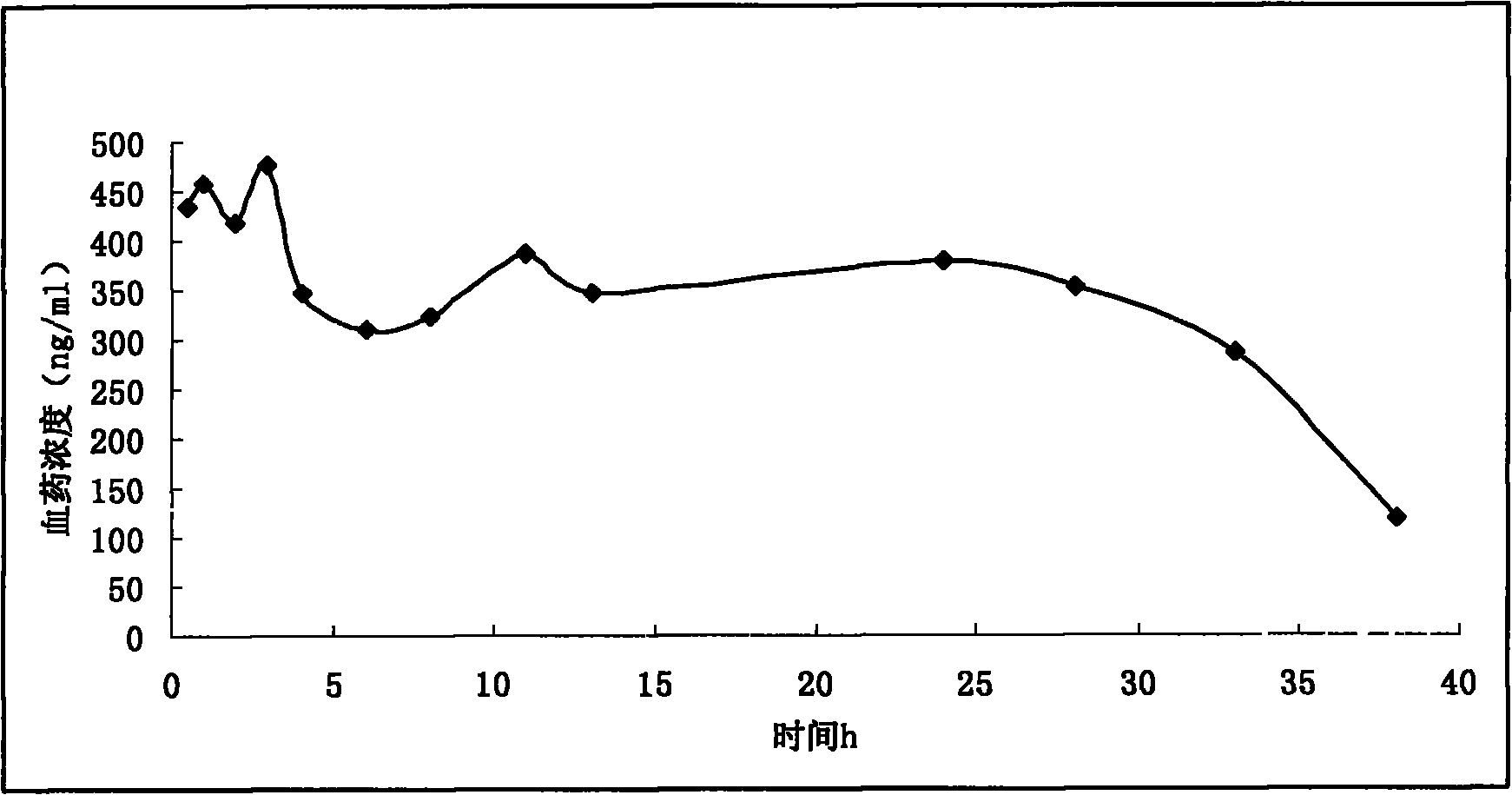
Morphine sulfate sustained/controlled-release suppository and preparation method thereof
A morphine sulfate and suppository technology, which is applied in the directions of suppository delivery, pharmaceutical formulations, and non-active ingredients medical preparations, etc., can solve the problems of unstable blood drug concentration, inability to achieve sustained and controlled release, and short effect time, and reduce the dosage of drugs. Frequency of medication, reliable curative effect, and the effect of reducing the dose
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] The morphine sulfate sustained-release suppository provided in this embodiment contains the following percentages by weight:
[0047] Morphine sulfate 3.48%;
[0048] Poloxamer 188 24.00%;
[0049] Poloxamer 407 26.40%;
[0050] BHT 0.02%;
[0051] Glycerin 40.50%
[0052] Azone 4.80%;
[0053] Carbomer 0.80%.
[0054] The preparation method of above-mentioned morphine sulfate suppository, comprises the steps:
[0055] A. Pass the main drug morphine sulfate through a 180 or more mesh sieve; the auxiliary materials include F68, F127, carbomer, 2,6-di-tert-butyl p-cresol (BHT), and the auxiliary materials pass through a 100 mesh or more mesh sieve respectively ,spare;
[0056] B. F68, F127, BHT, morphine sulfate, carbomer, stir and mix well, heat to 60-80°C to melt, stir and mix well;
[0057] C. Stir the molten substrate in step B at 60-75°C, add glycerin and azone and keep warm and stir evenly;
[0058] D. Stir the mixture in step C at a constant temperature of 6...
Embodiment 2
[0074] The morphine sulfate sustained-release suppository provided in this embodiment contains the following percentages by weight:
[0075] Morphine sulfate 5.94%;
[0076] Poloxamer 188 28.05%;
[0077] Poloxamer 407 25.25%;
[0078] BHT 0.02%;
[0079] Glycerin 35.84%
[0080] Azone 4.20%;
[0081] Carbomer 0.70%.
[0082] The preparation method of above-mentioned morphine sulfate suppository, comprises the steps:
[0083] A. Pass the main drug morphine sulfate through a 180 or more mesh sieve; the auxiliary materials include F68, F127, carbomer, 2,6-di-tert-butyl p-cresol (BHT), and the auxiliary materials pass through a 100 mesh or more mesh sieve respectively ,spare;
[0084] B. F68, F127, BHT, morphine sulfate, and carbomer are stirred and mixed, then heated to 70°C to melt, and stirred again;
[0085] C. Add glycerin and azone to the melted substrate in step B under stirring at 40-45°C and keep warm and stir evenly;
[0086] D. Stir the mixture in step C to a ...
Embodiment 3
[0105] The morphine sulfate sustained-release suppository provided in this embodiment contains the following percentages by weight:
[0106] Morphine sulfate 7.70%;
[0107] Poloxamer 188 21.76%;
[0108] Poloxamer 407 28.29%;
[0109] BHT 0.02%;
[0110] Glycerin 37.16%
[0111] Azone 4.35%;
[0112] Carbomer 0.72%
[0113] The preparation method of above-mentioned morphine sulfate suppository, comprises the steps:
[0114] A. Pass the main drug morphine sulfate through a 180 or more mesh sieve; the auxiliary materials include F68, F127, carbomer, 2,6-di-tert-butyl p-cresol (BHT), and the auxiliary materials pass through a 100 mesh or more mesh sieve respectively ,spare;
[0115] B. F68, F127, BHT, morphine sulfate, and carbomer are stirred and mixed, then heated to 70°C to melt, and stirred again;
[0116] C. Add glycerin and azone to the melted substrate in step B under stirring at 40-45°C and keep warm and stir evenly;
[0117] D. Stir the mixture in step C at a c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com