Preparation method and application of osteoporotegerin-heat-shock protein 65-fused protein
A technology of fusion protein and protein, which is applied in the direction of hybrid peptide, bone disease, drug combination, etc., can solve the problem of no effect of synovial inflammation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
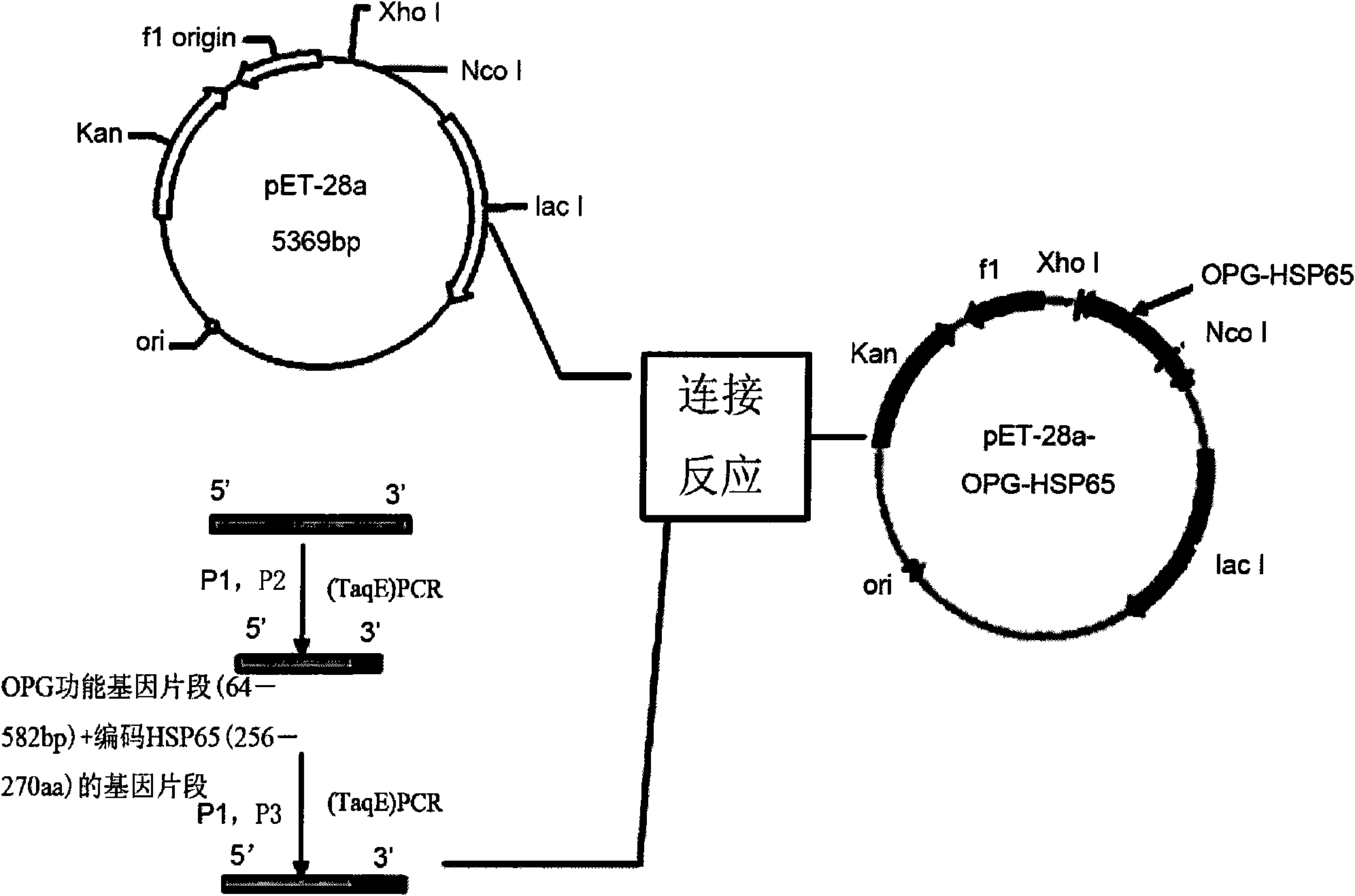
[0047] Example 1 Construction of OPG-HSP65 fusion protein prokaryotic expression vector
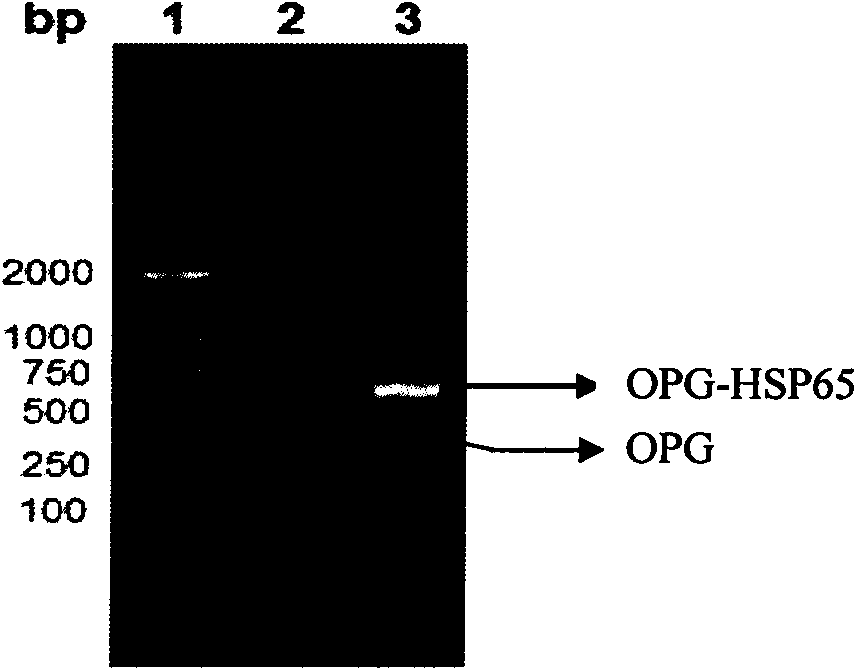
[0048] According to the gene sequence of the OPG-HSP65 recombinant fragment (OPG P22-194, HSP65P256-270) and the characteristics of the pET-28a expression vector, three primers were designed: the upstream primer P1 and the downstream primers P2 and P3, which retained the His of pET-28a Tag, with the recombinant plasmid pGEM-TEasy-OPG as a template, the MTB256-270aa gene sequence was designed into the primers, and the gene fragments of the two were connected by two PCR methods, and the TCC flexible fragment was introduced into the downstream primer P2, so that The spatial conformation of OPG-HSP65 recombinant protein was formed correctly. PCR reaction conditions: denaturation at 95°C for 5 min, cycled 30 times according to the following parameters: denaturation at 94°C for 40 s, annealing at 53°C for 35 s, extension at 68°C for 1 min, and finally extension at 72°C for 7 min. PCR products ...
Embodiment 2
[0049] Example 2 Induced expression of OPG-HSP65 fusion protein in Escherichia coli
[0050] Transform Escherichia coli E. coli BL21 (DE3) competent cells with the recombinant clone plasmids identified by restriction enzyme digestion, pick a single colony and inoculate in 25 mL LB medium (containing 25 mg / L kanamycin), and culture overnight at 37°C with shaking. The next day, take 50ml of the overnight culture and transfer it to 1L LB medium (containing 25mg / L kanamycin), culture at 37°C until OD 600 To 0.5, add IPTG to make the final concentration 0.5mmol / L, continue shaking culture at 30°C for 5h, and then centrifuge to collect the bacteria.
Embodiment 3
[0051] Example 3 Purification and renaturation of OPG-HSP65 fusion protein in Escherichia coli
[0052] Positive clones were cultured at 0.5mmol / L IPTG at 30°C for 5h, centrifuged at 6000r / min for 15min at 4°C, collected the cells, and washed with pre-cooled 20mmol / L Tris-HCL (pH=7.9) buffer , Wash the cells with pre-cooled 20mmol / LTris-HCL (pH 7.9) buffer. Add 10 times the volume of bacterial cell protein lysis solution per gram of wet bacteria to resuspend, add lysozyme to 1mg / ml, and add protease inhibitor PMSF to 1mmol / L at the same time, place on ice for 30min, then ultrasonically lyse the bacteria in an ice bath After 10 min, the precipitated inclusion bodies were collected by centrifugation. Wash twice with 2mol / L and 3mol / L urea inclusion body washing solution respectively, then dissolve the inclusion body with 4mol / L urea denaturing solution, centrifuge at 12000r / min for 45min at 4°C, and take the supernatant through a 0.22μm micropore The denatured solution of the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com