Preparation and use of OPG-HSP70 fusion protein
A technology of fusion proteins and proteins, which can be applied in the fields of peptide/protein components, chemical instruments and methods, medical preparations containing active ingredients, etc., and can solve the problems of ineffectiveness of synovial inflammation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
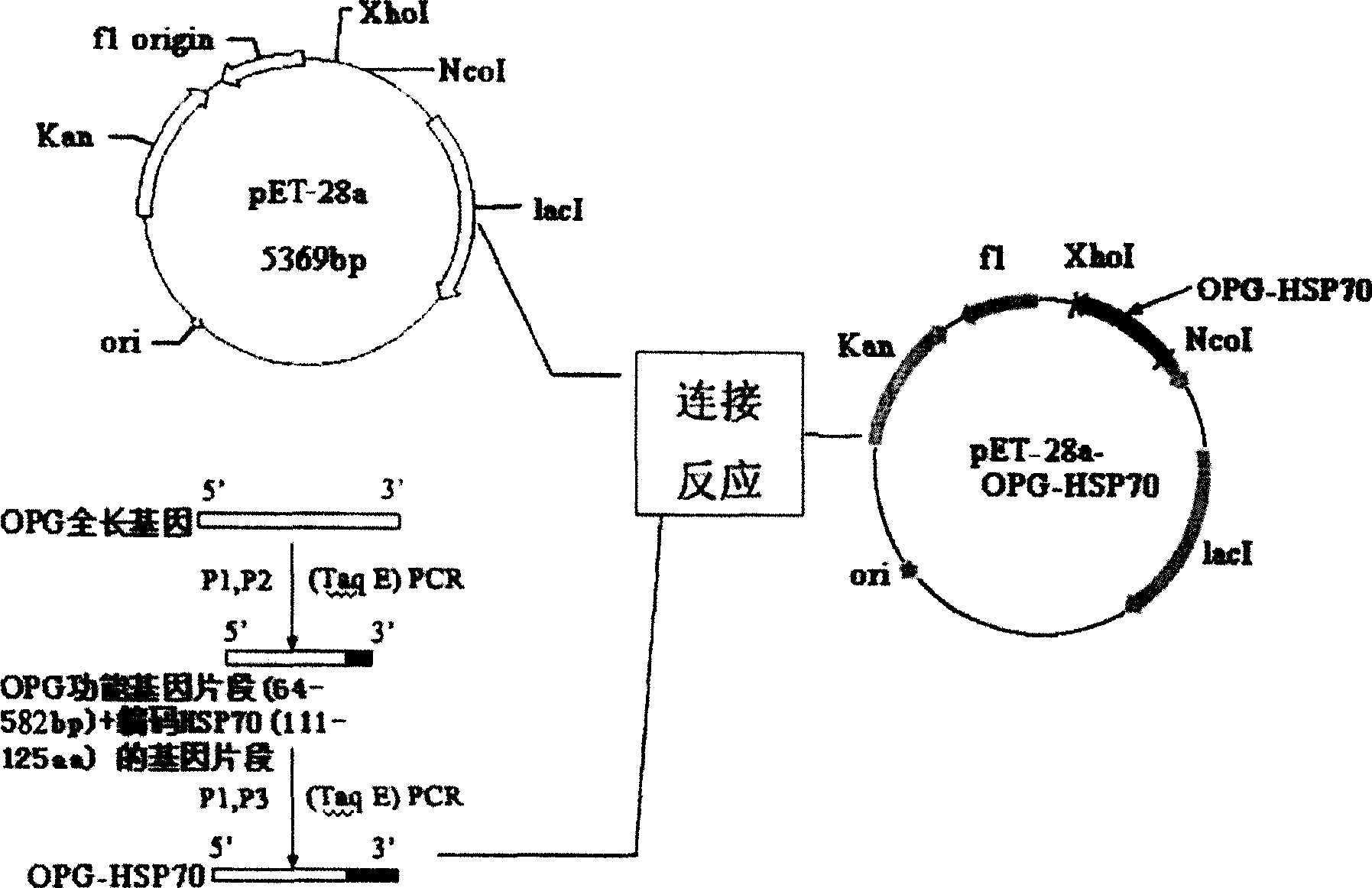
[0050] Cloning of embodiment 1 OPG full-length gene
[0051] According to the sequence of the human OPG coding region cDNA reported in the literature (GenBank No. NM_002546), two oligonucleotide primers for the OPG coding region cDNA were designed, and the human osteosarcoma cell line MG63 was cultivated to the early logarithmic growth stage, and rhBMP-2 (in The final concentration in the culture medium reaches 100ug / L), and after continuing to cultivate to the logarithmic growth phase, the cells are counted and centrifuged to collect the cells. Total cellular RNA was extracted according to the instructions of the Trizol Reagent total RNA extraction reagent. According to the operating steps of the MMLV first-strand cDNA synthesis kit, the first-strand cDNA was synthesized using oligo(dT) as a primer. Take 8uL of the reverse transcription product as a template, under the action of TaqDNA polymerase, carry out PCR with P1 and P2 primers respectively, the reaction conditions are...
Embodiment 2
[0052] Example 2 Construction of OPG-HSP70 fusion protein prokaryotic expression vector
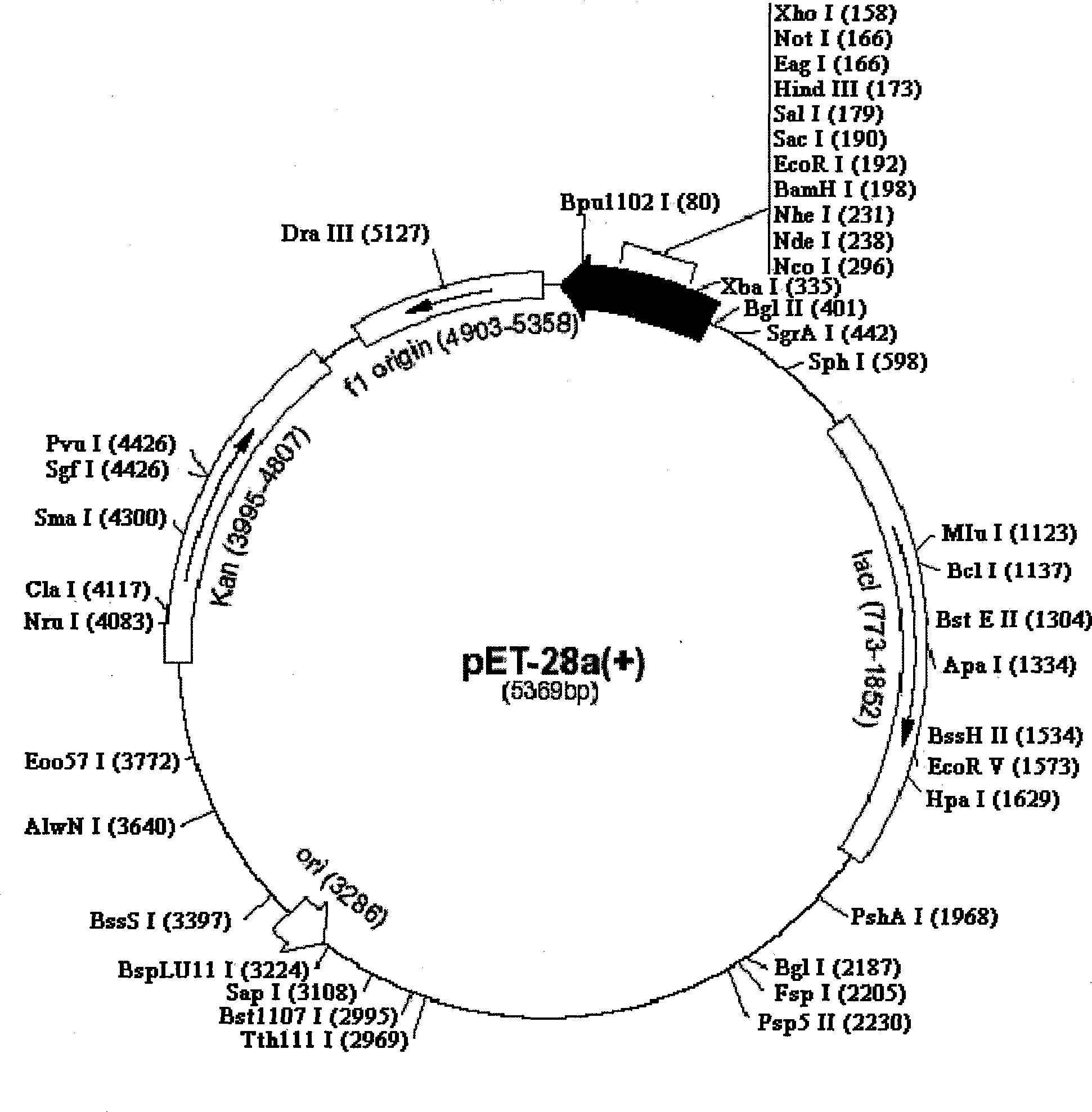
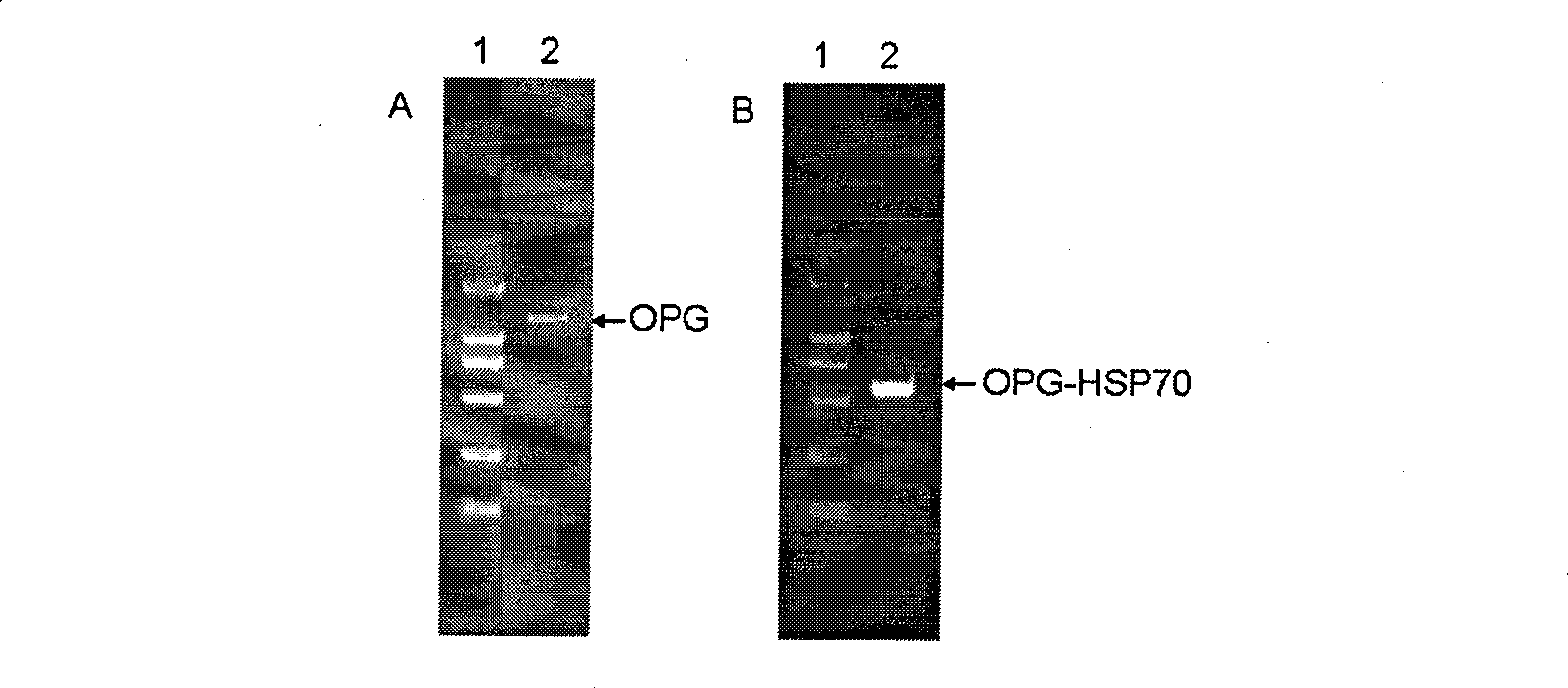
[0053] The recombinant plasmid pGEMT-Easy-OPG with correct sequencing was used as a template, and P3 and P4 were used as primers for the first PCR amplification, and then the PCR product was used as a template, and P3 and P5 were used as primers for the second PCR amplification. The OPG-HSP70 fusion gene was obtained. After purification, the PCR product was subjected to a double-enzyme digestion reaction with the prokaryotic expression vector pET-28a using Nco I and Xho I restriction endonucleases. After digestion, the carrier and the target DNA were purified and connected, transformed into E. coli DH5a competent cells, and a single colony was selected and inoculated in a 5 mL LB medium (containing 25 mg / L kanamycin) Erlenmeyer flask, 37 °C shaking overnight. The bacteria were collected, a small amount of plasmid was extracted, and then identified by Nco I and Xho I double enzyme digest...
Embodiment 3
[0054] Example 3 Induced expression of OPG-HSP70 fusion protein in Escherichia coli
[0055] Transform Escherichia coli E.coli BL21 (DE3) competent cells with the recombinant clone plasmids identified by restriction enzyme digestion, and select a single colony to inoculate in 5 mL 2×YT medium (containing 25 mg / L kanamycin), and shake overnight at 37°C. After the transfer at 1:50 the next day, culture at 37°C for 2 hours, add IPTG to make the final concentration 0.1mmol / L, continue shaking culture at 30°C for 5 hours, and collect the bacteria by centrifugation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com